Obstet Gynecol Sci.
2015 Mar;58(2):106-111. 10.5468/ogs.2015.58.2.106.
Clinical significance of mismatch repair genes immunohistochemical expression of complex endometrial hyperplasia
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. minkyukim@skku.edu
- KMID: 2314033
- DOI: http://doi.org/10.5468/ogs.2015.58.2.106
Abstract
OBJECTIVE
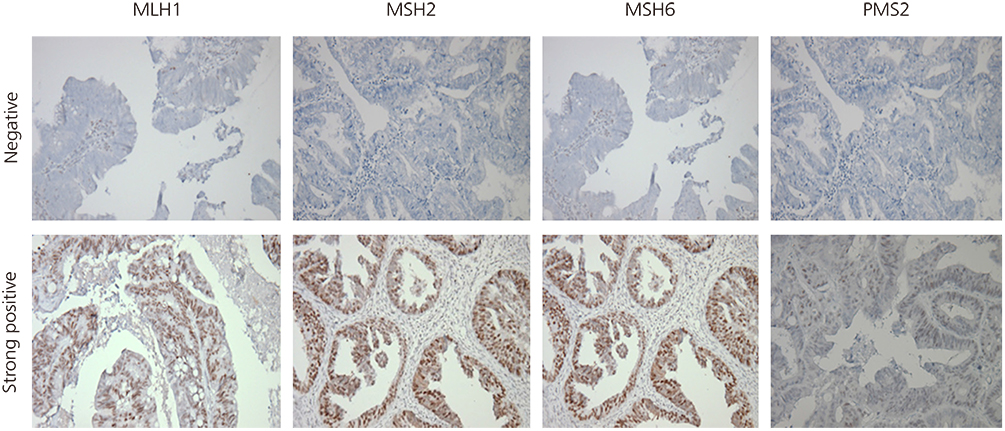
Women with Lynch syndrome have an increased risk of developing colorectal and gynecologic malignancies such as endometrial cancer. Complex hyperplasia has about a 30% risk of developing into endometrial cancer. The aim of this study was to determine the genetic risk for developing endometrial cancer by immunohistochemical staining of premalignant lesions for mutL homolog 1, mutS homolog 2, mutS homolog 6, and postmeiotic segregation increased 2.
METHODS
Twenty cases (n=20) were selected from among patients with available sample blocks for analysis. Clinical information was obtained from medical chart review. Immunohistochemical staining was performed for all of the tumor blocks. Staining was scored based on the intensity (intensity score 0-3) .
RESULTS
Among the 20 cases of complex endometrial hyperplasia, 11 (55%) patients showed loss of expression of at least one of the following proteins: mutL homolog 1, mutS homolog 2, mutS homolog 6, or postmeiotic segregation increased 2. Seven (35%) patients were negative for the expression of two or more proteins, and one patient (5%) was negative for the expression of all four proteins.
CONCLUSION
More than half of the patients showed loss of expression of at least one mismatch repair protein in our study population. Genetic risk counseling and further tests are recommended for these patients.
MeSH Terms
Figure
Reference
-
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.2. Emons G, Fleckenstein G, Hinney B, Huschmand A, Heyl W. Hormonal interactions in endometrial cancer. Endocr Relat Cancer. 2000; 7:227–242.3. Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000; 13:295–308.4. Lacey JV Jr, Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J Clin Oncol. 2010; 28:788–792.5. Gruber SB, Thompson WD. A population-based study of endometrial cancer and familial risk in younger women. Cancer and Steroid Hormone Study Group. Cancer Epidemiol Biomarkers Prev. 1996; 5:411–417.6. Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control. 2009; 16:14–22.7. Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999; 116:1453–1456.8. Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993; 75:1027–1038.9. Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994; 263:1625–1629.10. Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993; 75:1215–1225.11. Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994; 368:258–261.12. Burgart LJ. Testing for defective DNA mismatch repair in colorectal carcinoma: a practical guide. Arch Pathol Lab Med. 2005; 129:1385–1389.13. Hendriks YM, Jagmohan-Changur S, van der Klift HM, Morreau H, van Puijenbroek M, Tops C, et al. Heterozygous mutations in PMS2 cause hereditary nonpolyposis colorectal carcinoma (Lynch syndrome). Gastroenterology. 2006; 130:312–322.14. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–268.15. Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, et al. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996; 2:169–174.16. Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993; 53:5100–5103.17. Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996; 56:4836–4840.18. Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997; 57:4749–4756.19. Leach FS, Polyak K, Burrell M, Johnson KA, Hill D, Dunlop MG, et al. Expression of the human mismatch repair gene hMSH2 in normal and neoplastic tissues. Cancer Res. 1996; 56:235–240.20. Marcus VA, Madlensky L, Gryfe R, Kim H, So K, Millar A, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumors. Am J Surg Pathol. 1999; 23:1248–1255.21. Tafe LJ, Riggs ER, Tsongalis GJ. Lynch syndrome presenting as endometrial cancer. Clin Chem. 2014; 60:111–121.22. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013; 45:15–21.23. Resnick KE, Hampel H, Fishel R, Cohn DE. Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecol Oncol. 2009; 114:128–134.24. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008; 10:293–300.25. Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006; 354:261–269.26. Shia J, Holck S, Depetris G, Greenson JK, Klimstra DS. Lynch syndrome-associated neoplasms: a discussion on histopathology and immunohistochemistry. Fam Cancer. 2013; 12:241–260.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of cyclooxygenase-2 and p53 expression in normal endometrium, endometrial hyperplasia and endometrial cancer
- An Immunohistochemical Study of the Relationships between Estrogen and Progesterone Receptors and Proliferating Cell Nuclear Antigen in Endometrial Hyperplasia and Adenocarcinoma
- Expression of p53 Gene in the Endometrial Hyperplasia and Andenocarcinoma
- Expression Pattern of DNA Mismatch Repair Genes in Tumors of Microsatellite Mutator Phenotype
- Prognostic Significance of Immunohistochemical MSH2 Expression in Prostate Cancer