Nutr Res Pract.
2016 Feb;10(1):3-10. 10.4162/nrp.2016.10.1.3.
Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-Autophagy Pathway
- Affiliations
-
- 1Department of Biomedical Sciences, College of Medicine, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Korea. oshin@korea.ac.kr
- 2BK21 Plus Graduate Program, Department of Animal Science and Institute of Rare Earth for Biological Application , Chonbuk National University, Jeonju 54896, Korea.
- 3Institute of Natural Medicine, University of Toyama, Toyama, Japan.
- 4Department of Microbiology, College of Medicine, Korea University, Seoul 02841, Korea.
- KMID: 2313894
- DOI: http://doi.org/10.4162/nrp.2016.10.1.3
Abstract
- BACKGROUND/OBJECTIVES
Oligonol, mainly found in lychee fruit, is an antioxidant polyphenolic compound which has been shown to have anti-inflammatory and anti-cancer properties. The detailed mechanisms by which oligonol may act as an anti-aging molecule have not been determined.
MATERIALS/METHODS
In this study, we evaluated the ability of oligonol to modulate sirtuin (SIRT) expression in human lung epithelial (A549) cells. Oligonol was added to A549 cells and reactive oxygen species production, mitochondrial superoxide formation, and p21 protein levels were measured. Signaling pathways activated upon oligonol treatment were also determined by western blotting. Furthermore, the anti-aging effect of oligonol was evaluated ex vivo in mouse splenocytes and in vivo in Caenorhabditis elegans.
RESULTS
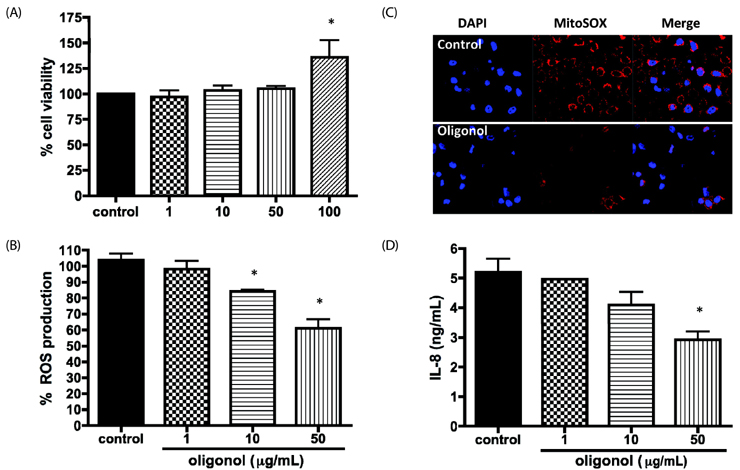
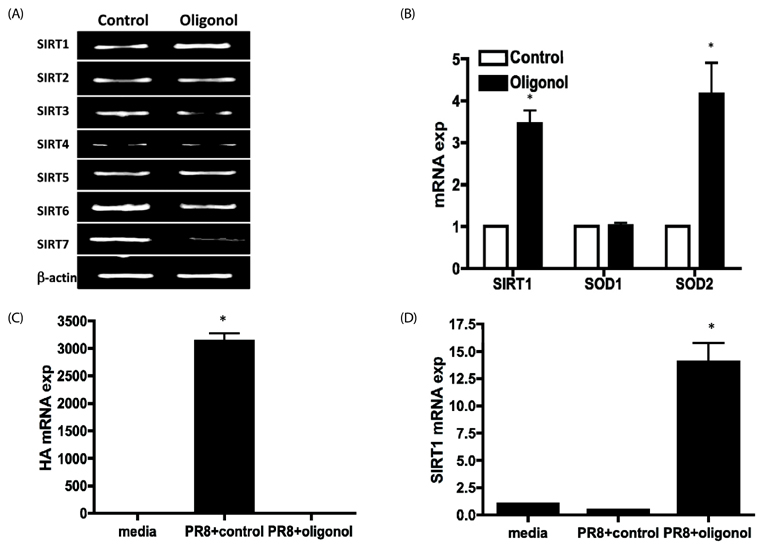
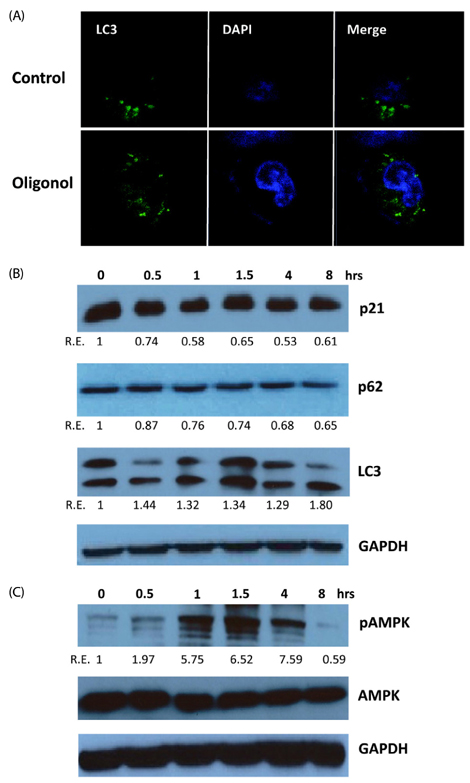
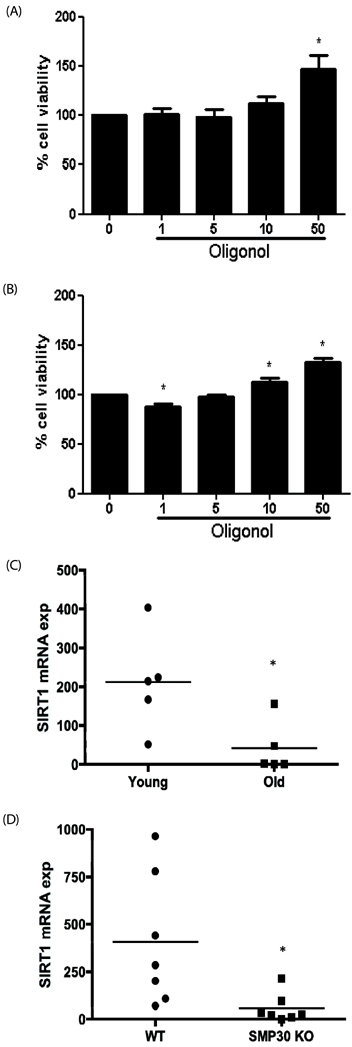
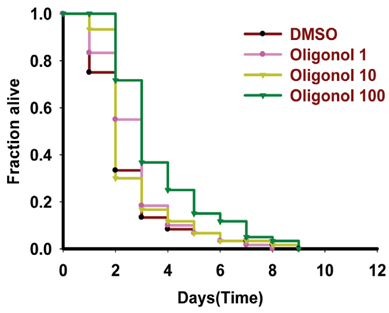
Oligonol specifically induced the expression of SIRT1, whose activity is linked to gene expression, metabolic control, and healthy aging. In response to influenza virus infection of A549 cells, oligonol treatment significantly up-regulated SIRT1 expression and down-regulated viral hemagglutinin expression. Oligonol treatment also resulted in the activation of autophagy pathways and the phosphorylation of AMP-activated protein kinase (AMPK). Furthermore, oligonol-treated spleen lymphocytes from old mice showed increased cell proliferation, and mRNA levels of SIRT1 in the lungs of old mice were significantly lower than those in the lungs of young mice. Additionally, in vivo lethality assay revealed that oligonol extended the lifespan of C. elegans infected with lethal Vibrio cholerae.
CONCLUSIONS
These data demonstrated that oligonol may act as an anti-aging molecule by modulating SIRT1/autophagy/AMPK pathways.
Keyword
MeSH Terms
-
Aging
AMP-Activated Protein Kinases
Animals
Autophagy
Blotting, Western
Caenorhabditis elegans
Cell Proliferation
Fruit
Gene Expression
Hemagglutinins, Viral
Humans
Litchi
Lung
Lymphocytes
Mice
Orthomyxoviridae
Phosphorylation
Reactive Oxygen Species
RNA, Messenger
Spleen
Superoxides
Vibrio cholerae
AMP-Activated Protein Kinases
Hemagglutinins, Viral
RNA, Messenger
Reactive Oxygen Species
Superoxides
Figure
Reference
-
1. Fujii H, Sun B, Nishioka H, Hirose A, Aruoma OI. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem Toxicol. 2007; 45:378–387.
Article2. Tomobe K, Fujii H, Sun B, Nishioka H, Aruoma OI. Modulation of infection-induced inflammation and locomotive deficit and longevity in senescence-accelerated mice-prone (SAMP8) model by the oligomerized polyphenol Oligonol. Biomed Pharmacother. 2007; 61:427–434.
Article3. Kundu JK, Hwang DM, Lee JC, Chang EJ, Shin YK, Fujii H, Sun B, Surh YJ. Inhibitory effects of oligonol on phorbol ester-induced tumor promotion and COX-2 expression in mouse skin: NF-kappaB and C/EBP as potential targets. Cancer Lett. 2009; 273:86–97.
Article4. Lee SJ, Chung IM, Kim MY, Park KD, Park WH, Moon HI. Inhibition of lung metastasis in mice by oligonol. Phytother Res. 2009; 23:1043–1046.
Article5. Park CH, Noh JS, Fujii H, Roh SS, Song YO, Choi JS, Chung HY, Yokozawa T. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates gluco-lipotoxicity-mediated renal disorder in type 2 diabetic db/db mice. Drug Discov Ther. 2015; 9:13–22.
Article6. Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011; 76:81–90.
Article7. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003; 425:191–196.
Article8. Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010; 5:253–295.
Article9. Korkina LG, Pastore S, Dellambra E, De Luca C. New molecular and cellular targets for chemoprevention and treatment of skin tumors by plant polyphenols: a critical review. Curr Med Chem. 2013; 20:852–868.
Article10. Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011; 19:163–174.
Article11. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase SIRT1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008; 105:3374–3379.
Article12. Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012; 11:230–241.
Article13. Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011; 146:682–695.
Article14. Fujii H, Nakagawa T, Nishioka H, Sato E, Hirose A, Ueno Y, Sun B, Yokozawa T, Nonaka G. Preparation, characterization, and antioxidative effects of oligomeric proanthocyanidin-L-cysteine complexes. J Agric Food Chem. 2007; 55:1525–1531.
Article15. Shin OS, Yanagihara R, Song JW. Distinct innate immune responses in human macrophages and endothelial cells infected with shrew-borne hantaviruses. Virology. 2012; 434:43–49.
Article16. Jung KJ, Lee EK, Kim SJ, Song CW, Maruyama N, Ishigami A, Kim ND, Im DS, Yu BP, Chung HY. Anti-inflammatory activity of SMP30 modulates NF-kappaB through protein tyrosine kinase/phosphatase balance. J Mol Med (Berl). 2015; 93:343–356.
Article17. Kim JI, Park S, Lee I, Lee S, Shin S, Won Y, Hwang MW, Bae JY, Heo J, Hyun HE, Jun H, Lim SS, Park MS. GFP-expressing influenza A virus for evaluation of the efficacy of antiviral agents. J Microbiol. 2012; 50:359–362.
Article18. Kim Y, Mylonakis E. Caenorhabditis elegans immune conditioning with the probiotic bacterium Lactobacillus acidophilus strain NCFM enhances gram-positive immune responses. Infect Immun. 2012; 80:2500–2508.
Article19. Kim HI, Kim JA, Choi EJ, Harris JB, Jeong SY, Son SJ, Kim Y, Shin OS. In vitro and in vivo antimicrobial efficacy of natural plantderived compounds against Vibrio cholerae of O1 El Tor Inaba serotype. Biosci Biotechnol Biochem. 2015; 79:475–483.
Article20. Gangehei L, Ali M, Zhang W, Chen Z, Wakame K, Haidari M. Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine. 2010; 17:1047–1056.
Article21. Koyuncu E, Budayeva HG, Miteva YV, Ricci DP, Silhavy TJ, Shenk T, Cristea IM. Sirtuins are evolutionarily conserved viral restriction factors. MBio. 2014; 5(pii):e02249-14.
Article22. Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010; 5:e15394.
Article23. Jin J, Iakova P, Jiang Y, Medrano EE, Timchenko NA. The reduction of SIRT1 in livers of old mice leads to impaired body homeostasis and to inhibition of liver proliferation. Hepatology. 2011; 54:989–998.
Article24. Maruyama N, Ishigami A, Kondo Y. Pathophysiological significance of senescence marker protein-30. Geriatr Gerontol Int. 2010; 10:Suppl 1. S88–S98.
Article25. Ermolaeva MA, Schumacher B. Insights from the worm: the C. elegans model for innate immunity. Semin Immunol. 2014; 26:303–309.
Article26. Cinar HN, Kothary M, Datta AR, Tall BD, Sprando R, Bilecen K, Yildiz F, McCardell B. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One. 2010; 5:e11558.
Article27. Sahu SN, Lewis J, Patel I, Bozdag S, Lee JH, LeClerc JE, Cinar HN. Genomic analysis of immune response against Vibrio cholerae hemolysin in Caenorhabditis elegans. PLoS One. 2012; 7:e38200.
Article28. Fujii H, Yokozawa T, Kim YA, Tohda C, Nonaka G. Protective effect of grape seed polyphenols against high glucose-induced oxidative stress. Biosci Biotechnol Biochem. 2006; 70:2104–2111.
Article29. Sakurai T, Nishioka H, Fujii H, Nakano N, Kizaki T, Radak Z, Izawa T, Haga S, Ohno H. Antioxidative effects of a new lychee fruit- derived polyphenol mixture, oligonol, converted into a low- molecular form in adipocytes. Biosci Biotechnol Biochem. 2008; 72:463–476.
Article30. Fujii H, Nishioka H, Wakame K, Magnuson BA, Roberts A. Acute, subchronic and genotoxicity studies conducted with Oligonol, an oligomerized polyphenol formulated from lychee and green tea extracts. Food Chem Toxicol. 2008; 46:3553–3562.
Article31. Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012; 69:2999–3013.
Article32. Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012; 48:158–167.
Article33. Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013; 6:19.
Article34. West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011; 472:476–480.
Article35. Rutanen J, Yaluri N, Modi S, Pihlajamäki J, Vänttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M, Sinclair DA, Elliott P, Westphal C, Auwerx J, Laakso M. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010; 59:829–835.
Article36. Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011; 1215:138–143.
Article37. Park JY, Kim Y, Im JA, You S, Lee H. Inhibition of Adipogenesis by Oligonol through Akt-mTOR Inhibition in 3T3-L1 Adipocytes. Evid Based Complement Alternat Med. 2014; 2014:895272.
Article38. Park CH, Yokozawa T, Noh JS. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates diabetes-induced renal damage through the advanced glycation end product-related pathway in db/db mice. J Nutr. 2014; 144:1150–1157.
Article39. Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008; 7:3669–3679.
Article40. Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009; 458:1056–1060.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential
- Aquatide Activation of SIRT1 Reduces Cellular Senescence through a SIRT1-FOXO1-Autophagy Axis
- Cellular NAD⺠Level: A Key Determinant of Mitochondrial Quality and Health
- Extracellular Ubiquitin Enhances Autophagy and Inhibits Mitochondrial Apoptosis Pathway to Protect Neurons Against Spinal Cord Ischemic Injury via CXCR4
- Dual Roles of Autophagy and Their Potential Drugs for Improving Cancer Therapeutics