Nutr Res Pract.
2015 Apr;9(2):117-122. 10.4162/nrp.2015.9.2.117.
Curcumin utilizes the anti-inflammatory response pathway to protect the intestine against bacterial invasion
- Affiliations
-
- 1Division of GI Cell Biology, Boston Children's Hospital, USA.
- 2Department of Food and Nutrition, Hannam University, 461-6 Jeonmin-dong, Yuseong-gu, Daejeon 305-811, Korea. eunmi_park@hnu.kr
- KMID: 2313818
- DOI: http://doi.org/10.4162/nrp.2015.9.2.117
Abstract
- BACKGROUND/OBJECTIVES
Curcumin, a major component of the Curcuma species, contains antioxidant and anti-inflammatory properties. Although it was found to induce apoptosis in cancer cells, the functional role of curcumin as well as its molecular mechanism in anti-inflammatory response, particularly in intestinal cells, has been less investigated. The intestine epithelial barrier is the first barrier and the most important location for the substrate coming from the lumen of the gut.
SUBJECTS/METHODS
We administered curcumin treatment in the human intestinal epithelial cell lines, T84 and Caco-2. We examined endoplasmic reticulum (ER) stress response by thapsigargin, qPCR of XBP1 and BiP, electrophysiology by wild-type cholera toxin in the cells.
RESULTS
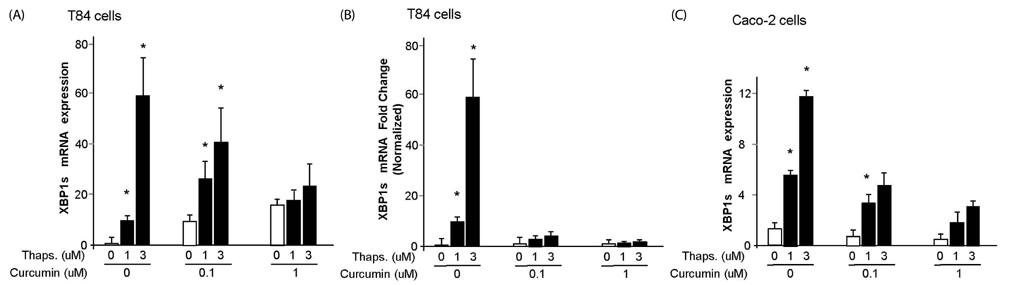
In this study, we showed that curcumin treatment reduces ER stress and thereby decreases inflammatory response in human intestinal epithelial cells. In addition, curcumin confers protection without damaging the membrane tight junction or actin skeleton change in intestine epithelial cells. Therefore, curcumin treatment protects the gut from bacterial invasion via reduction of ER stress and anti-inflammatory response in intestinal epithelial cells.
CONCLUSIONS
Taken together, our data demonstrate the important role of curcumin in protecting the intestine by modulating ER stress and inflammatory response post intoxication.
Keyword
MeSH Terms
Figure
Reference
-
1. Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008; 132:24–26.
Article2. Cho JA, Lee AH, Platzer B, Cross BC, Gardner BM, De Luca H, Luong P, Harding HP, Glimcher LH, Walter P, Fiebiger E, Ron D, Kagan JC, Lencer WI. The unfolded protein response element IRE1alpha senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe. 2013; 13:558–569.
Article3. Ji JL, Huang XF, Zhu HL. Curcumin and its formulations: potential anti-cancer agents. Anticancer Agents Med Chem. 2012; 12:210–218.
Article4. Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, Ohno T, Tsurumi H, Tanaka T, Moriwaki H. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer. 2012; 64:72–79.
Article5. Zeng Z, Zhan L, Liao H, Chen L, Lv X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-kappaB signaling pathway. Planta Med. 2013; 79:102–109.
Article6. Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, Yu XP, Peng DD, Su L, Xiao B, Zhang ZS. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-kappaB activation. PLoS One. 2010; 5:e12969.7. Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005; 11:1747–1752.
Article8. Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999; 20:445–451.
Article9. Zhang M, Deng C, Zheng J, Xia J, Sheng D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol. 2006; 6:1233–1242.
Article10. Bounaama A, Djerdjouri B, Laroche-Clary A, Le Morvan V, Robert J. Short curcumin treatment modulates oxidative stress, arginase activity, aberrant crypt foci, and TGF-beta1 and HES-1 transcripts in 1,2-dimethylhydrazine-colon carcinogenesis in mice. Toxicology. 2012; 302:308–317.
Article11. Epstein J, Docena G, MacDonald TT, Sanderson IR. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr. 2010; 103:824–832.
Article12. Moon DO, Jin CY, Lee JD, Choi YH, Ahn SC, Lee CM, Jeong SC, Park YM, Kim GY. Curcumin decreases binding of Shiga-like toxin-1B on human intestinal epithelial cell line HT29 stimulated with TNF-alpha and IL-1beta: suppression of p38, JNK and NF-kappaB p65 as potential targets. Biol Pharm Bull. 2006; 29:1470–1475.
Article13. Lencer WI, Delp C, Neutra MR, Madara JL. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992; 117:1197–1209.
Article14. Glimcher LH, Lindvall O, Aguirre V, Topalian SL, Musunuru K, Fauci AS. Translating research into therapies. Cell. 2012; 148:1077–1078.
Article15. Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010; 140:859–870.
Article16. Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008; 5:e54.
Article17. DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012; 246:379–400.18. Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012; 56:704–713.
Article19. Karin M. Tracking the road from inflammation to cancer: the critical role of IkappaB kinase (IKK). Harvey Lect. 2006-2007; 102:133–151.20. Karin M. The IkappaB kinase-a bridge between inflammation and cancer. Cell Res. 2008; 18:334–342.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of heat shock protein 70 in regulation of anti-inflammatory response to curcumin in 3T3-L1 adipocytes
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- 6-Shogaol and 10-Shogaol Synergize Curcumin in Ameliorating Proinflammatory Mediators via the Modulation of TLR4/TRAF6/ MAPK and NFκB Translocation
- Anti-Migration and Anti-Invasion Effects of Curcumin via Suppression of Fascin Expression in Glioblastoma Cells
- Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice