Nutr Res Pract.
2023 Jun;17(3):397-407. 10.4162/nrp.2023.17.3.397.
Role of heat shock protein 70 in regulation of anti-inflammatory response to curcumin in 3T3-L1 adipocytes
- Affiliations
-
- 1Major of Food and Nutrition, Division of Applied Food System, Seoul Women’s University, Seoul 01797, Korea
- 2Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA
- KMID: 2542838
- DOI: http://doi.org/10.4162/nrp.2023.17.3.397
Abstract
- BACKGROUND/OBJECTIVES
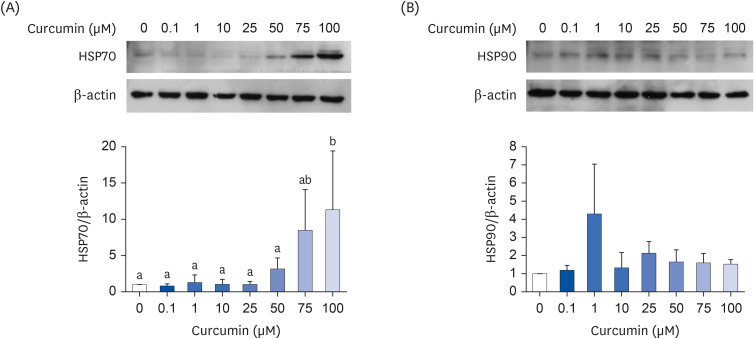
Curcumin is a well-known phytochemical with antiinflammatory effects. Heat shock protein (HSP) 70, an intracellular chaperone, inhibits proinflammatory signaling activation. Although curcumin has been shown to induce HSP70 expression in various cell types, whether HSP70 mediates the anti-inflammatory effects of curcumin in mature adipocytes remains unclear.
MATERIALS/METHODS
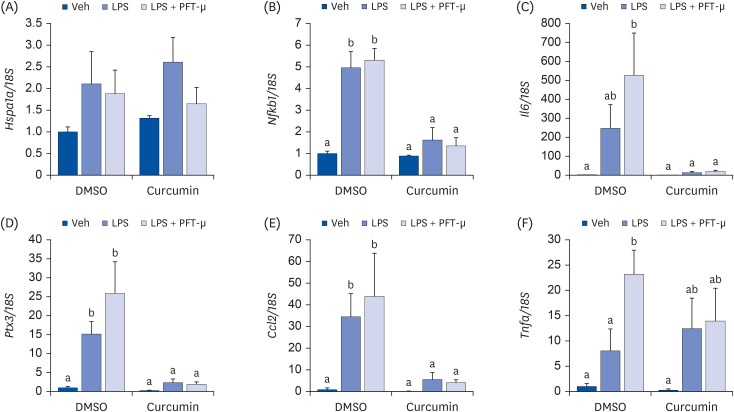
To assess the role of HSP70 in regulating the anti-inflammatory response to curcumin in adipocytes, fully differentiated 3T3-L1 adipocytes were treated with curcumin, lipopolysaccharide (LPS), and/or the HSP70 inhibitor pifithrin-μ (PFT-μ). The expression levels of HSP70 and proinflammatory cytokines were then measured.
RESULTS
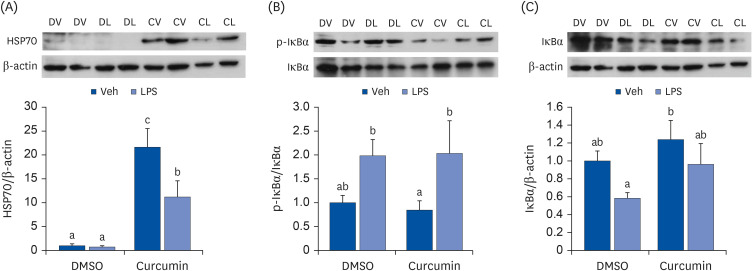
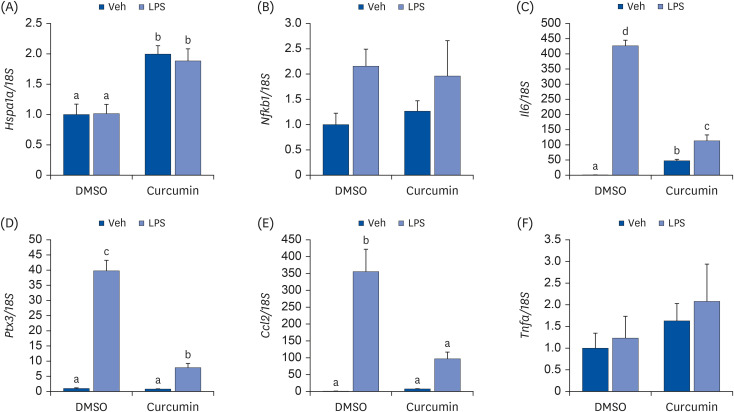
Curcumin upregulated HSP70 expression at both protein and mRNA levels and attenuated LPS-induced Il6, Ptx3, and Ccl2> mRNA upregulation. PFT-μ tended to exacerbate the LPS-induced upregulation of Il6, Ptx3,Ccl2>, and Tnfa mRNA expression. However, on curcumin pretreatment, the tendency of PFT-μ to upregulate LPS-induced proinflammatory cytokine expression decreased or disappeared.
CONCLUSION
These results indicate that HSP70 is involved in the regulation of inflammatory responses but may not be crucial for the anti-inflammatory effects of curcumin in 3T3-L1 adipocytes.
Figure
Reference
-
1. Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009; 57:4467–4477. PMID: 19489612.2. Prasad S, Aggarwal B. Chronic diseases caused by chronic inflammation require chronic treatment: anti-inflammatory role of dietary spices. J Clin Cell Immunol. 2014; 5:238.3. Afman L, Milenkovic D, Roche HM. Nutritional aspects of metabolic inflammation in relation to health--insights from transcriptomic biomarkers in PBMC of fatty acids and polyphenols. Mol Nutr Food Res. 2014; 58:1708–1720. PMID: 24449395.4. Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008; 10:236–246. PMID: 18543123.5. Si H, Liu D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J Nutr Biochem. 2014; 25:581–591. PMID: 24742470.6. Shehzad A, Ha T, Subhan F, Lee YS. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr. 2011; 50:151–161. PMID: 21442412.7. Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr Metab (Lond). 2008; 5:17. PMID: 18549505.8. Jabczyk M, Nowak J, Hudzik B, Zubelewicz-Szkodzińska B. Curcumin in metabolic health and disease. Nutrients. 2021; 13:4440. PMID: 34959992.9. Jalali M, Mahmoodi M, Mosallanezhad Z, Jalali R, Imanieh MH, Moosavian SP. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020; 48:102283. PMID: 31987259.10. Chien YJ, Chang CY, Wu MY, Chen CH, Horng YS, Wu HC. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: systematic review with meta-analysis and trial sequential analysis. Nutrients. 2021; 13:684. PMID: 33669954.11. Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010; 9:43. PMID: 20937162.12. Chen YC, Tsai SH, Shen SC, Lin JK, Lee WR. Alternative activation of extracellular signal-regulated protein kinases in curcumin and arsenite-induced HSP70 gene expression in human colorectal carcinoma cells. Eur J Cell Biol. 2001; 80:213–221. PMID: 11322385.13. Teiten MH, Reuter S, Schmucker S, Dicato M, Diederich M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 2009; 279:145–154. PMID: 19246153.14. Guo M, Xu W, Yamamoto Y, Suzuki T. Curcumin increases heat shock protein 70 expression via different signaling pathways in intestinal epithelial cells. Arch Biochem Biophys. 2021; 707:108938. PMID: 34051214.15. Wang J, Lee J, Liem D, Ping P. HSPA5 gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene. 2017; 618:14–23. PMID: 28286085.16. Kim JY, Yenari MA. The immune modulating properties of the heat shock proteins after brain injury. Anat Cell Biol. 2013; 46:1–7. PMID: 23560231.17. Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKKγ and impairing NF-κB survival signaling. Genes Dev. 2004; 18:1466–1481. PMID: 15198984.18. Ferat-Osorio E, Sánchez-Anaya A, Gutiérrez-Mendoza M, Boscó-Gárate I, Wong-Baeza I, Pastelin-Palacios R, Pedraza-Alva G, Bonifaz LC, Cortés-Reynosa P, Pérez-Salazar E, et al. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J Inflamm (Lond). 2014; 11:19. PMID: 25053922.19. Green A, Krause J, Rumberger JM. Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomedicine. 2014; 21:118–122. PMID: 24060216.20. Wang N, Wang F, Gao Y, Yin P, Pan C, Liu W, Zhou Z, Wang J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci. 2016; 132:192–200. PMID: 27840063.21. Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008; 149:3549–3558. PMID: 18403477.22. Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, Lu H, Fantus IG, Jin T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 2012; 7:e28784. PMID: 22253696.23. Shin SK, Ha TY, McGregor RA, Choi MS. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol Nutr Food Res. 2011; 55:1829–1840. PMID: 22058071.24. Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, Li XX, Sun CH. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res. 2013; 57:1569–1577. PMID: 22930403.25. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012; 35:2121–2127. PMID: 22773702.26. Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, de Bittencourt PI Jr. Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity (Silver Spring). 2015; 23:120–129. PMID: 25292174.27. Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011; 300:E894–E901. PMID: 21325107.28. Gu Y, Chen J, Wang T, Zhou C, Liu Z, Ma L. Hsp70 inducer, 17-allylamino-demethoxygeldanamycin, provides neuroprotection via anti-inflammatory effects in a rat model of traumatic brain injury. Exp Ther Med. 2016; 12:3767–3772. PMID: 28101166.29. Tulapurkar ME, Ramarathnam A, Hasday JD, Singh IS. Bacterial lipopolysaccharide augments febrile-range hyperthermia-induced heat shock protein 70 expression and extracellular release in human THP1 cells. PLoS One. 2015; 10:e0118010. PMID: 25659128.30. Zhang Y, Ma X, Jiang D, Chen J, Jia H, Wu Z, Kim IH, Yang Y. Glycine attenuates lipopolysaccharide-induced acute lung injury by regulating NLRP3 inflammasome and NRF2 signaling. Nutrients. 2020; 12:611. PMID: 32110933.31. Bernardini C, Zannoni A, Turba ME, Fantinati P, Tamanini C, Bacci ML, Forni M. Heat shock protein 70, heat shock protein 32, and vascular endothelial growth factor production and their effects on lipopolysaccharide-induced apoptosis in porcine aortic endothelial cells. Cell Stress Chaperones. 2005; 10:340–348. PMID: 16333987.32. Wu S, Yano S, Chen J, Hisanaga A, Sakao K, He X, He J, Hou DX. Polyphenols from Lonicera caerulea L. berry inhibit LPS-induced inflammation through dual modulation of inflammatory and antioxidant mediators. J Agric Food Chem. 2017; 65:5133–5141. PMID: 28573848.33. Gupta A, Cooper ZA, Tulapurkar ME, Potla R, Maity T, Hasday JD, Singh IS. Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release. J Biol Chem. 2013; 288:2756–2766. PMID: 23212905.34. Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009; 36:15–27. PMID: 19818706.35. Ishaq M, Ojha R, Sharma K, Sharma G, Singh SK, Majumdar S. Functional inhibition of Hsp70 by pifithrin-μ switches Gambogic acid induced caspase dependent cell death to caspase independent cell death in human bladder cancer cells. Biochim Biophys Acta. 2016; 1863:2560–2573. PMID: 27395830.36. Sheng L, Tang T, Liu Y, Ma Y, Wang Z, Tao H, Zhang Y, Qi Z. Inducible HSP70 antagonizes cisplatin\xe2\x80\x91induced cell apoptosis through inhibition of the MAPK signaling pathway in HGC\xe2\x80\x9127 cells. Int J Mol Med. 2018; 42:2089–2097. PMID: 30066840.37. Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: molecular targets of thunder god vine. Biochem Biophys Res Commun. 2010; 394:439–442. PMID: 20226165.38. Paimela T, Hyttinen JM, Viiri J, Ryhänen T, Karjalainen RO, Salminen A, Kaarniranta K. Celastrol regulates innate immunity response via NF-κB and Hsp70 in human retinal pigment epithelial cells. Pharmacol Res. 2011; 64:501–508. PMID: 21683142.39. Ohnishi K, Nakahata E, Irie K, Murakami A. Zerumbone, an electrophilic sesquiterpene, induces cellular proteo-stress leading to activation of ubiquitin-proteasome system and autophagy. Biochem Biophys Res Commun. 2013; 430:616–622. PMID: 23219816.40. Igarashi Y, Ohnishi K, Irie K, Murakami A. Possible contribution of zerumbone-induced proteo-stress to its anti-inflammatory functions via the activation of heat shock factor 1. PLoS One. 2016; 11:e0161282. PMID: 27536885.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- Effects of mulberry fruit juice powder on inflammation and microRNA-132/143 regulation in 3T3-L1 adipocytes
- Korean Curcuma longa L. induces lipolysis and regulates leptin in adipocyte cells and rats