Nutr Res Pract.
2014 Apr;8(2):158-164.
Peanut sprouts extract (Arachis hypogaea L.) has anti-obesity effects by controlling the protein expressions of PPARgamma and adiponectin of adipose tissue in rats fed high-fat diet
- Affiliations
-
- 1Department of Food and Nutrition, Eulji University, 553 Sanseong-Daero,Seongnam-Si, Gyeonggi 461-632, Korea.
- 2Department of Food Science and Nutrition, Dankook University, 152 Jukjeon-Ro, Suji-Gu, Yongin-Si, Gyeonggi 448-701, Korea. wkkim@dankook.ac.kr
Abstract
- BACKGROUD/OBEJECTIVES: This study aims to find out the effects of peanut sprout extracts on weight controls and protein expressions of transcription factors related to adipocyte differentiation and adipocytokine in rats under high-fat diets.
MATERIALS/METHODS
Four week-old Sparague-Dawley (SD) were assigned to 4 groups; normal-fat (NF) diets (7% fat diet), high-fat (HF) diets (20% fat diet), high fat diets with low peanut sprout extract (HF + PSEL) diet (20% fat and 0.025% peanut sprout extract), and high fat diets with high peanut sprout extract (HF + PSEH) diet (20% fat and 0.05% peanut sprout extract). Body weight changes, lipid profiles in adipose tissue, and the mRNA protein expressions, such as peroxisome proliferator-activated receptor gamma (PPARgamma), CCAAT element binding protein alpha (C/EBP alpha), leptin, and adiponectin, were determined.
RESULTS
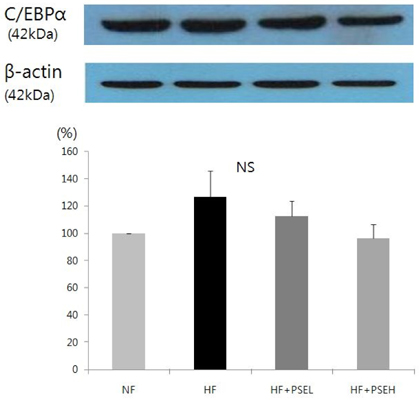
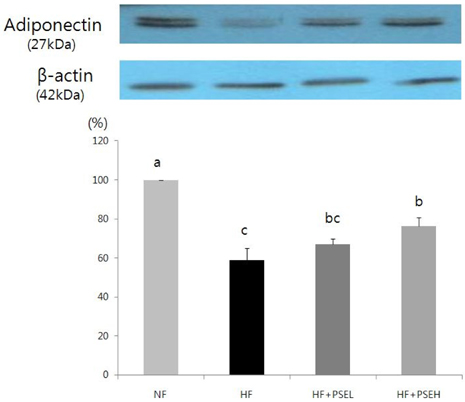
After 9 weeks of feeding, the HF + PSEH group had significantly less weight gains than the HF group (P < 0.05). However, the total dietary intakes or food efficiency ratios among groups were not significantly different. The weight of epididymal fat in HF + PSEH group, 3.61 +/- 0.5 g, or HF + PSEL group, 3.80 +/- 0.7 g, was significantly lower than the HF group, 4.39 +/- 0.4g, (P < 0.05). Total lipids and total cholesterol in adipose tissue were significantly decreased in HF + PSEH group compared to those in the HF group, respectively (P < 0.05). PSEH supplementation caused AST and ALT levels to decrease when it compared to HF group, but it was not statistically significant. The protein expression of PPARgamma in HF + PSEH group was significantly lower than the HF group (P < 0.05). Comparing with the HF group, the protein expression of adiponectin in HF + PSEH group was significantly increased (P < 0.05). The protein expressions of C/EBP alpha and leptin in HF + PSEH group were lower than the HF group, but it was not statistical significant.
CONCLUSIONS
In conclusion, peanut sprout extract has anti-obesity effect by lowering the expressions of PPARgamma which regulates the expression of adiponectin.
Keyword
MeSH Terms
Figure
Reference
-
1. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013; 9:191–200.2. Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J). 2007; 83:S192–S203.
Article3. Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006; 580:2917–2921.
Article4. Fajas L, Fruchart JC, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998; 10:165–173.
Article5. Grimaldi PA. The roles of PPARs in adipocyte differentiation. Prog Lipid Res. 2001; 40:269–281.
Article6. Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998; 138:619–620.
Article7. Rocha KK, Souza GA, Ebaid GX, Seiva FR, Cataneo AC, Novelli EL. Resveratrol toxicity: effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem Toxicol. 2009; 47:1362–1367.
Article8. Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008; 29:698–706.
Article9. Aubin MC, Lajoie C, Clément R, Gosselin H, Calderone A, Perrault LP. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther. 2008; 325:961–968.
Article10. Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009; 77:1053–1063.
Article11. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008; 8:157–168.
Article12. Szkudelska K, Nogowski L, Szkudelski T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009; 113:17–24.
Article13. Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012; 6:499–504.
Article14. Rural Development Administratio, National Institute of Crop Science (KR). Rural Development Administration News [Internet]. Suwon: Rural Development Administration;2010. cited 2010 Oct 14. Available from: http://rda.korea.kr/gonews.15. Kang HI, Kim JY, Park KW, Kang JS, Choi MR, Moon KD, Seo KI. Resveratrol content and nutritional components in peanut sprouts. Korean J Food Preserv. 2010; 17:384–390.16. Kang HI, Kim JY, Kwon SJ, Park KW, Kang JS, Seo KI. Antioxidative effects of peanut sprout extracts. J Korean Soc Food Sci Nutr. 2010; 39:941–946.
Article17. Kim HJ, Kang JS, Park HR, Hwang YI. Neuroprotective effects of methanolic extracts from peanut sprouts. J Life Sci. 2010; 20:253–259.
Article18. Lee SE, Park CH, Bang JK, Seong NS, Chung TY. Comparison on antioxidant potential of several peanut varieties. J Korean Soc Food Sci Nutr. 2004; 33:941–945.
Article19. Wang KH, Lai YH, Chang JC, Ko TF, Shyu SL, Chiou RY. Germination of peanut kernels to enhance resveratrol biosynthesis and prepare sprouts as a functional vegetable. J Agric Food Chem. 2005; 53:242–246.
Article20. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article21. Frings CS, Dunn RT. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Pathol. 1970; 53:89–91.
Article22. Lin BS, Lien TF, Chao MR, Lai TY, Chang JC, Chou SJ, Liao HF, Chiou RY. Toxicological and nutraceutical assessments of peanut sprouts as daily supplements to feed Sprague-Dawley rats for 18 weeks. J Sci Food Agric. 2008; 88:2201–2207.
Article23. Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008; 367:190–194.
Article24. Park SH, Park TS, Cha YS. Grape seed extract (Vitis vinifera) partially reverses high fat diet-induced obesity in C57BL/6J mice. Nutr Res Pract. 2008; 2:227–233.
Article25. Rayalam S, Della-Fera MA, Yang JY, Park HJ, Ambati S, Baile CA. Resveratrol potentiates genistein's antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J Nutr. 2007; 137:2668–2673.
Article26. Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, Baile CA. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008; 11:773–783.
Article27. Kim HS, Kim TW, Kim DJ, Hwang HJ, Lee HJ, Choe M. Effects of natural plants supplementation on adipocyte size of the epididymal fat pads in rats. J Korean Soc Food Sci Nutr. 2007; 36:419–423.
Article28. Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPARγ gene expression by nutrition and obesity in rodents. J Clin Invest. 1996; 97:2553–2561.29. Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Werman A, Pories WJ, Caro JF, Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997; 99:2416–2422.
Article30. White UA, Stephens JM. Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol. 2010; 318:10–14.
Article31. Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005; 54:3358–3370.
Article32. Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004; 314:151–158.
Article33. Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002; 25:971–976.
Article34. Yamamoto Y, Hirose H, Saito I, Tomita M, Taniyama M, Matsubara K, Okazaki Y, Ishii T, Nishikai K, Saruta T. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond). 2002; 103:137–142.
Article35. Barnea M, Shamay A, Stark AH, Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity (Silver Spring). 2006; 14:2145–2153.
Article36. Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008; 60:790–797.
Article37. Considine RV, Caro JF. Leptin: genes, concepts and clinical perspective. Horm Res. 1996; 46:249–256.
Article38. Szkudelska K, Nogowski L, Szkudelski T. The inhibitory effect of resveratrol on leptin secretion from rat adipocytes. Eur J Clin Invest. 2009; 39:899–905.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of the Sasa Borealis Leaves Extract on Plasma Adiponectin, Resistin, C-Reactive Protein and Homocysteine Levels in High Fat Diet-Induced Obese C57/BL6J Mice
- Anti-obesity effects of hot water extract from Wasabi (Wasabia japonica Matsum.) leaves in mice fed high-fat diets
- Anti-obesity effect of Korean Hamcho (Salicornia herbacea L.) powder on high-fat diet-induced obese rats
- Anti-obesity effects of Rapha diet(R) preparation in mice fed a high-fat diet
- Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet