Nutr Res Pract.
2014 Apr;8(2):146-150.
Naringenin stimulates cholecystokinin secretion in STC-1 cells
- Affiliations
-
- 1Division of Metabolism and Functionality Research, Korea Food Research Institute, 62 Road-1201 Anyang-Pangyo-Ro, Bundang, Sungnam, Gyeonggi 463-746, Korea. khyey@kfri.re.kr
Abstract
- BACKGROUND/OBJECTIVES
Cholecystokinin (CCK), a hormone or neuropeptide, is secreted in response to intraluminal nutrients by enteroendocrine I-cells of the intestine and has important physiological actions related to appetite regulation and satiety. The stimulation on CCK secretion from the intestine is of potential relevance for body weight management. Naringenin (4',5,7-trihydroxyflavanone) and its glycoside naringin (naringenin 7-rhamnoglucoside) have been reported to have many biological functions. In the current study, we investigated the question of whether naringenin and naringin could stimulate CCK secretion and then examined the mechanisms involved in CCK release.
MATERIALS/METHODS
STC-1 cells were used as a model of enteroendocrine cells. CCK release and changes in intracellular Ca2+ ([Ca2+]i) were measured after incubation of cells with naringenin and naringin for 1 h.
RESULTS
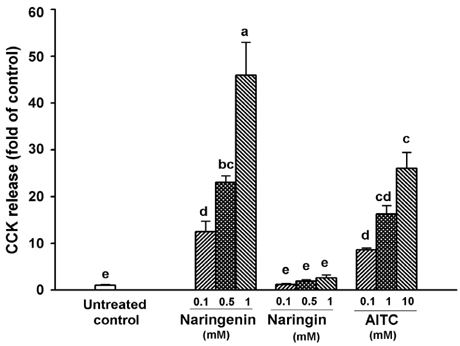
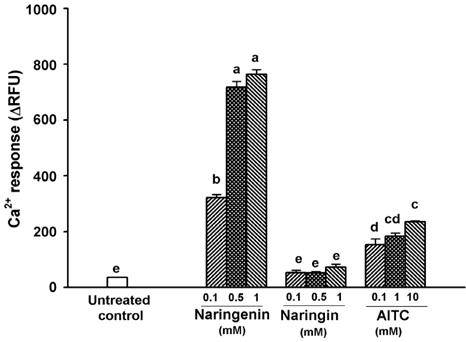
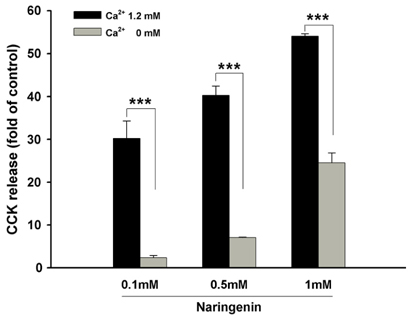
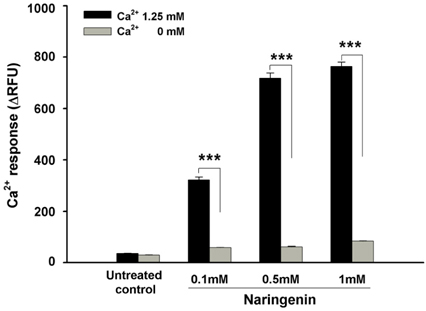
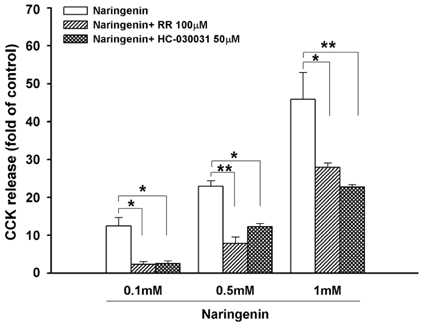
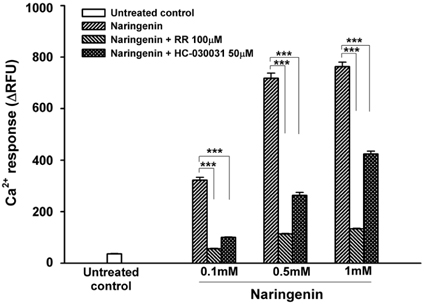
Naringenin caused significant (P < 0.05) stimulation of CCK secretion, but naringin did not. In addition, regarding the secretory mechanisms, naringenin-induced CCK secretion involved increases in [Ca2+]i, influx of extracellular Ca2+, at least in part, and activation of TRP channels, including TRPA1.
CONCLUSION
Findings of this study suggest that naringenin could have a role in appetite regulation and satiety.
Keyword
MeSH Terms
Figure
Reference
-
1. Hand KV, Bruen CM, O'Halloran F, Giblin L, Green BD. Acute and chronic effects of dietary fatty acids on cholecystokinin expression, storage and secretion in enteroendocrine STC-1 cells. Mol Nutr Food Res. 2010; 54:Suppl 1. S93–S103.
Article2. Dockray GJ. Chapter 4-Gastrointestinal hormones: gastrin, cholecystokinin, somatostatin, and ghrelin. In : Johnson LR, Barret KE, Gishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. New York (NY): Elsevier Academic Press;2006. Vol. 1:p. 91–120.3. Chen MC, Wu SV, Reeve JR Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006; 291:C726–C739.4. Purhonen AK, Louhivuori LM, Kiehne K, Kerman KE, Herzig KH. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008; 582:229–232.
Article5. Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res. 2004; 24:851–874.
Article6. Li XH, Xiong ZL, Lu S, Zhang Y, Li FM. Pharmacokinetics of naringin and its metabolite naringenin in rats after oral administration of Rhizoma drynariae extract assayed by UPLC-MS/MS. Chin J Nat Med. 2010; 8:40–46.
Article7. Van Acker FA, Schouten O, Haenen GR, van der Vijgh WJ, Bast A. Flavonoids can replace alpha-tocopherol as an antioxidant. FEBS Lett. 2000; 473:145–148.8. Guthrie N, Carroll KK. Inhibition of mammary cancer by citrus flavonoids. Adv Exp Med Biol. 1998; 439:227–236.
Article9. Mulvihill EE, Huff MW. Antiatherogenic properties of flavonoids: implications for cardiovascular health. Can J Cardiol. 2010; 26:Suppl A. 17A–21A.
Article10. Borradaile NM, de Dreu LE, Huff MW. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes. 2003; 52:2554–2561.
Article11. Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA, Huff MW. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009; 58:2198–2210.
Article12. Choi JS, Yokozawa T, Oura H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med. 1991; 57:208–211.
Article13. Ortiz-Andrade RR, Sánchez-Salgado JC, Navarrete-Vázquez G, Webster SP, Binnie M, García-Jiménez S, León-Rivera I, Cigarroa-Vázquez P, Villalobos-Molina R, Estrada-Soto S. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes Metab. 2008; 10:1097–1104.
Article14. Kannappan S, Anuradha CV. Naringenin enhances insulin-stimulated tyrosine phosphorylation and improves the cellular actions of insulin in a dietary model of metabolic syndrome. Eur J Nutr. 2010; 49:101–109.
Article15. Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008; 56:6185–6205.
Article16. Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr. 2004; 134:2499–2503.
Article17. Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 2006; 38:1134–1145.
Article18. Pu P, Gao DM, Mohamed S, Chen J, Zhang J, Zhou XY, Zhou NJ, Xie J, Jiang H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch Biochem Biophys. 2012; 518:61–70.
Article19. Kim HY, Park M, Kim K, Lee YM, Rhyu MR. Hesperetin stimulates cholecystokinin secretion in enteroendocrine STC-1 cells. Biomol Ther (Seoul). 2013; 21:121–125.
Article20. Zhu BW, Li DM, Zhou DY, Han S, Yang JF, Li T, Ye WX, Greeley GH. Structural analysis and CCK-releasing activity of a sulphated polysaccharide from abalone (Haliotis Discus Hannai Ino) viscera. Food Chem. 2011; 125:1273–1278.
Article21. Le Nevé B, Foltz M, Daniel H, Gouka R. The steroid glycoside H.g.-12 from Hoodia gordonii activates the human bitter receptor TAS2R14 and induces CCK release from HuTu-80 cells. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G1368–G1375.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hesperetin Stimulates Cholecystokinin Secretion in Enteroendocrine STC-1 Cells
- Secretory response of cultured acinar cells of rat pancreas to cholecystokinin
- The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose treated HepG2 cells
- Microbial Transformation of Two Prenylated Naringenins
- The Excitatory Effect of Cholecystokinin on Colonic Motor Function via Cholecystokinin1 Receptor