Nutr Res Pract.
2007 Sep;1(3):195-199.
Apoptotic effect of IP6 was not enhanced by co-treatment with myo-inositol in prostate carcinoma PC3 cells
- Affiliations
-
- 1Department of Food and Nutrition, Seoul National University, Seoul 151-742, Korea.
- 2Research Institute of Human Ecology, Seoul National University, Seoul 151-742, Korea. hye0414@snu.ac.kr
Abstract
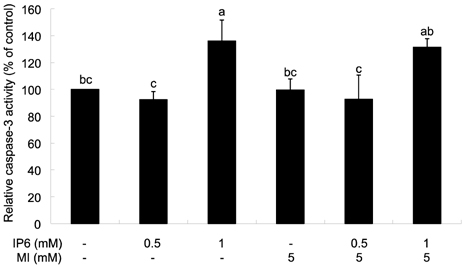
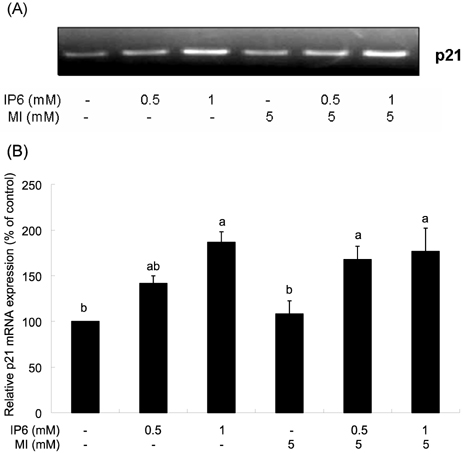
- Inositol hexaphosphate (IP6) is a major constituent of most cereals, legumes, nuts, oil seeds and soybean. Previous studies reported the anticancer effect of IP6 and suggested that co-treatment of IP6 with inositol may enhance anticancer effect of IP6. Although the anticancer effect of IP6 has been intensively studied, the combinational effect of IP6 and inositol and involved mechanisms are not well understood so far. In the present study, we investigated the effect of IP6 and myo-inositol (MI) on cell cycle regulation and apoptosis using PC3 prostate cancer cell lines. When cells were co-treated with IP6 and MI, the extent of cell growth inhibition was significantly increased than that by IP6 alone. To identify the effect of IP6 and MI on apoptosis, the activity of caspase-3 was measured. The caspase-3 activity was significantly increased when cells were treated with either IP6 alone or both IP6 and MI, with no significant enhancement by co-treatment. To investigate the effect of IP6 and MI of cell cycle arrest, we measured p21 mRNA expression in PC3 cells and observed significant increase in p21 mRNA by IP6. But synergistic regulation by co-treatment with IP6 and MI was not observed. In addition, there was no significant effect by co-treatment compared to IP6 treatment on the regulation of cell cycle progression although IP6 significantly changed cell cycle distribution in the presence of MI or not. Therefore, these findings support that IP6 has anticancer function by induction of apoptosis and regulation of cell cycle. However, synergistic effect by MI on cell cycle regulation and apoptosis was not observed in PC3 prostate cancer cells.
MeSH Terms
Figure
Reference
-
1. Agarwal C, Dhanalakshmi S, Singh R, Agarwal R. Inositol hexaphosphate inhibits growth and induces G1 arrest and apoptotic death of androgen-dependent human prostate carcinoma LNCaP cells. Neoplasia. 2004. 6:646–659.
Article2. Diallo JS, Peant B, Lessard L, Delvoye N, Le Page C, Mes-Masson AM, Saad F. An androgen-independent androgen receptor function protects from inositol hexakisphosphate toxicity in the PC3/PC3(AR) prostate cancer cell lines. Prostate. 2006. 66:1245–1256.
Article3. Estensen R, Wattenberg L. Studies of chemopreventive effects of myo-inositol on benzo[a]pyrene-induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993. 14:1975–1977.
Article4. Ferry S, Matsuda M, Yoshida H, Hirata M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFkB-mediated cell survival pathway. Carcinogenesis. 2002. 23:2031–2041.
Article5. Grases F, Simonet B, Vucenik I, Perello J, RM P, Shamsuddin A. Effects of exogenous inositol hexakisphosphate (InsP(6)) on the levels of InsP(6) and of inositol trisphosphate (InsP(3)) in malignant cells, tissues and biological fluids. Life Sci. 2002. 71:1535–1546.
Article6. Isaacs W, Carter B, Ewing C. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991. 51:4716–4720.7. Morgan DO. Principles of CDK regulation. Nature. 1995. 974:131–134.
Article8. Razzini G, Berrie C, Vignati S, Broggini M, Mascetta G, Brancaccio A, Falasca M. Novel functional PI 3-kinase antagonists inhibit cell growth and tumorigenicity in human cancer cell lines. FASEB J. 2000. 14:1179–1187.
Article9. Reddy N, Sathe S, Salunkhe D. Phytates in legumes and cereals. Adv Food Res. 1982. 28:1–92.
Article10. Sakamoto K, Venkatraman G, Shamsuddin A. Growth inhibition and differentiation of HT-29 cells in vitro by inositol hexaphosphate (phytic acid). Carcinogenesis. 1993. 14:1815–1819.
Article11. Sasakawa N, Sharif M, Hanley M. Metabolism and biological activities of inositol pentakisphosphate and inositol hexakisphosphate. Biochem Pharmacol. 1995. 50:137–146.
Article12. Shamsuddin A. Metabolism and cellular functions of IP6: a review. Anticancer Res. 1999. 19:3733–3736.13. Shamsuddin A, Ullah A, Chakravarthy A. Inositol and inositol hexaphosphate suppress cell proliferation and tumor formation in CD-1 mice. Carcinogenesis. 1989. 10:1461–1463.
Article14. Shamsuddin A, Vucenik I, Cole K. IP6: A novel anti-cancer agent. Life Sci. 1997. 61:343–354.
Article15. Shamsuddin A, Yang G, Vucenik I. Novel anti-cancer functions of IP6: growth inhibition and differentiation of human mammary cancer cell lines in vitro. Anticancer Res. 1996. 16:3287–3292.16. Singh RP, Agarwal C, Agarwal R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKI-CDK-cyclin and pRB-related protein-E2F complexes. Carcinogenesis. 2003. 24:555–563.
Article17. Singh RP, Sharma G, Mallikarjuna G, Dhanalakshm S, Agarwal C, Agarwal R. In vivo suppression of hormone-refractory prostate cancer growth by inositol hexaphosphate: induction of insulin-like growth factor binding protein-3 and inhibition of vascular endothelial growth factor. Clin Cancer Res. 2004. 10:244–250.
Article18. Venkateswaran V, Klotz LH, Fleshner NE. Selenium modulation of cell proliferation and cell cycle biomarkers in human prostate carcinoma cell lines. Cancer Res. 2002. 62:2540–2545.19. Vucenik I, Ramakrishna G, Tantivejkul K, Anderson L, Ramljak D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCdelta-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res Treat. 2005. 91:35–45.
Article20. Vucenik I, Shamsuddin A. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr. 2003. 133:3778S–3784S.
Article21. Vucenik I, Tantivejkul K, Zhang Z, Cole K, Saied I, Shamsuddin A. IP6 in treatment of liver cancer. I. IP6 inhibits growth and reverses transformed phenotype in HepG2 human liver cancer cell line. Anticancer Res. 1998a. 18:4083–4090.22. Vucenik I, Yang G, Shamsuddin A. Inositol hexaphosphate and inositol inhibit DMBA-induced rat mammary cancer. Carcinogenesis. 1995. 16:1055–1058.
Article23. Vucenik I, Zhang Z, Shamsuddin A. IP6 in treatment of liver cancer. II. Intra-tumoral injection of IP6 regresses pre-existing human liver cancer xenotransplanted in nude mice. Anticancer Res. 1998b. 18:4091–4096.24. Zi X, Singh R, Agarwal R. Impairment of erbB1 receptor and fluid-phase endocytosis and associated mitogenic signaling by inositol hexaphosphate in human prostate carcinoma DU145 cells. Carcinogenesis. 2000. 21:2225–2235.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anticancer Effects of IP6 in a Human Colon Carcinoma Cell Line in Nude Mice Xenografts
- Determination of Urinary Myo-/Chiro-Inositol Ratios from Korean Diabetes Patients
- Effect of Indole-3-Carbinol on Inhibition of MMP Activity via MAPK Signaling Pathway in Human Prostate Cancer Cell Line, PC3 Cells
- DRG2 levels in prostate cancer cell lines predict response to PARP inhibitor during docetaxel treatment
- The Effect of Transforming Growth Factor-Beta1 on Proliferation and Invasion of a Human Prostate Cancer Line, PC3 Cell, is Dependent on Seeding Density