Nat Prod Sci.
2016 Mar;22(1):53-59. 10.20307/nps.2016.22.1.53.
Dihydrobenzofuran Neolignans Isolated from Euonymus alatus Leaves and Twigs Attenuated Inflammatory Responses in the Activated RAW264.7 Macrophage Cells
- Affiliations
-
- 1Gyeongnam Department of Environment & Toxicology, Korea Institute of Toxicology, 17 Jegok-gil, Munsan-eup, Gyeongnam 660-844, Republic of Korea.
- 2College of Pharmacy, Pusan National University, Busan 609-735, Republic of Korea.
- 3College of Pharmacy and Research Institute of Pharmaceutical Science, Seoul National University, Seoul 151-742, Republic of Korea.
- 4Department of Agronomy & Medicinal Plant Resources, College of Life Sciences and Natural Resources, Gyeongnam National University of Science and Technology, Jinju 660-758, Republic of Korea. ejjeong@gntech.ac.kr
- KMID: 2312922
- DOI: http://doi.org/10.20307/nps.2016.22.1.53
Abstract
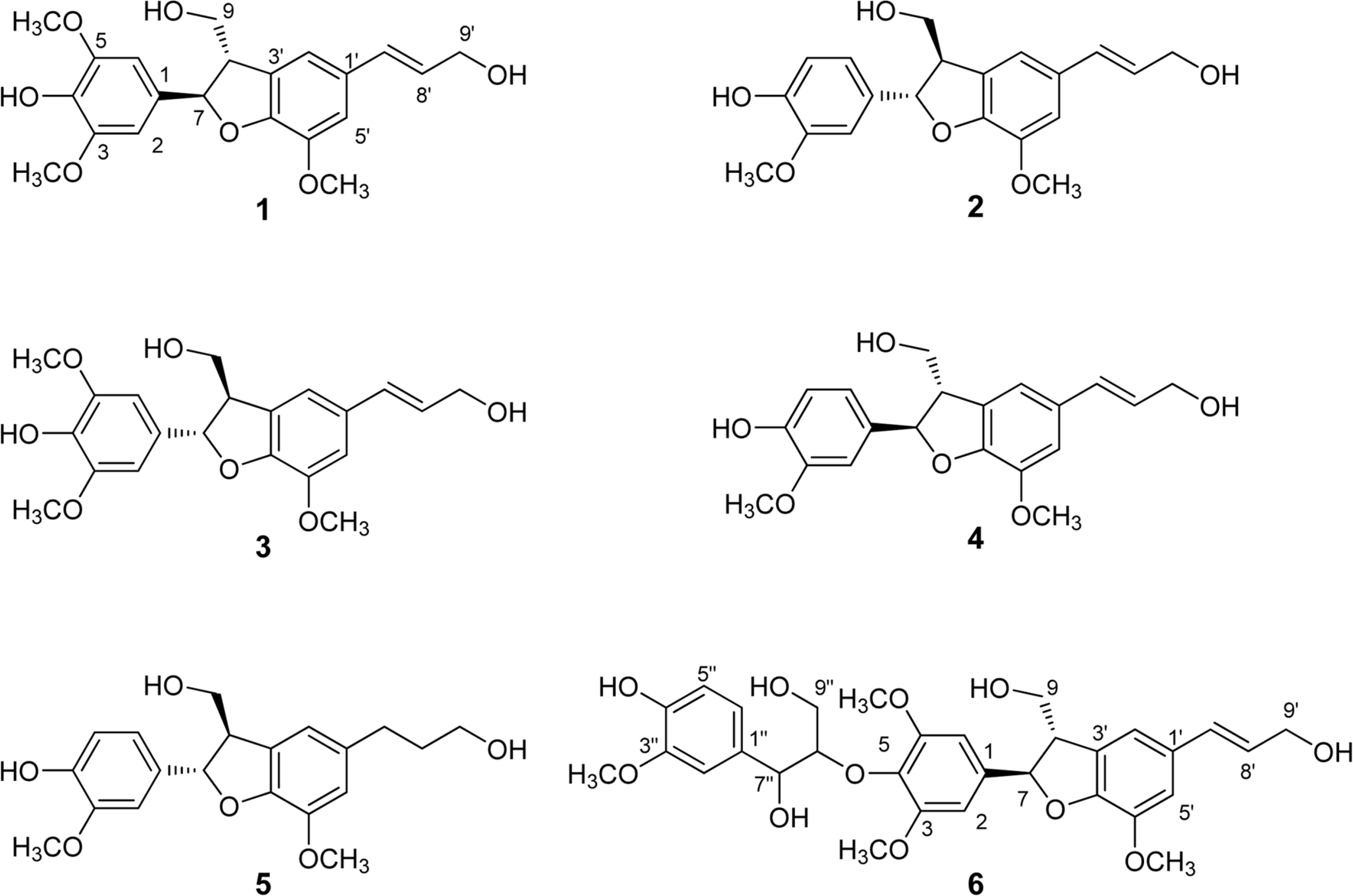
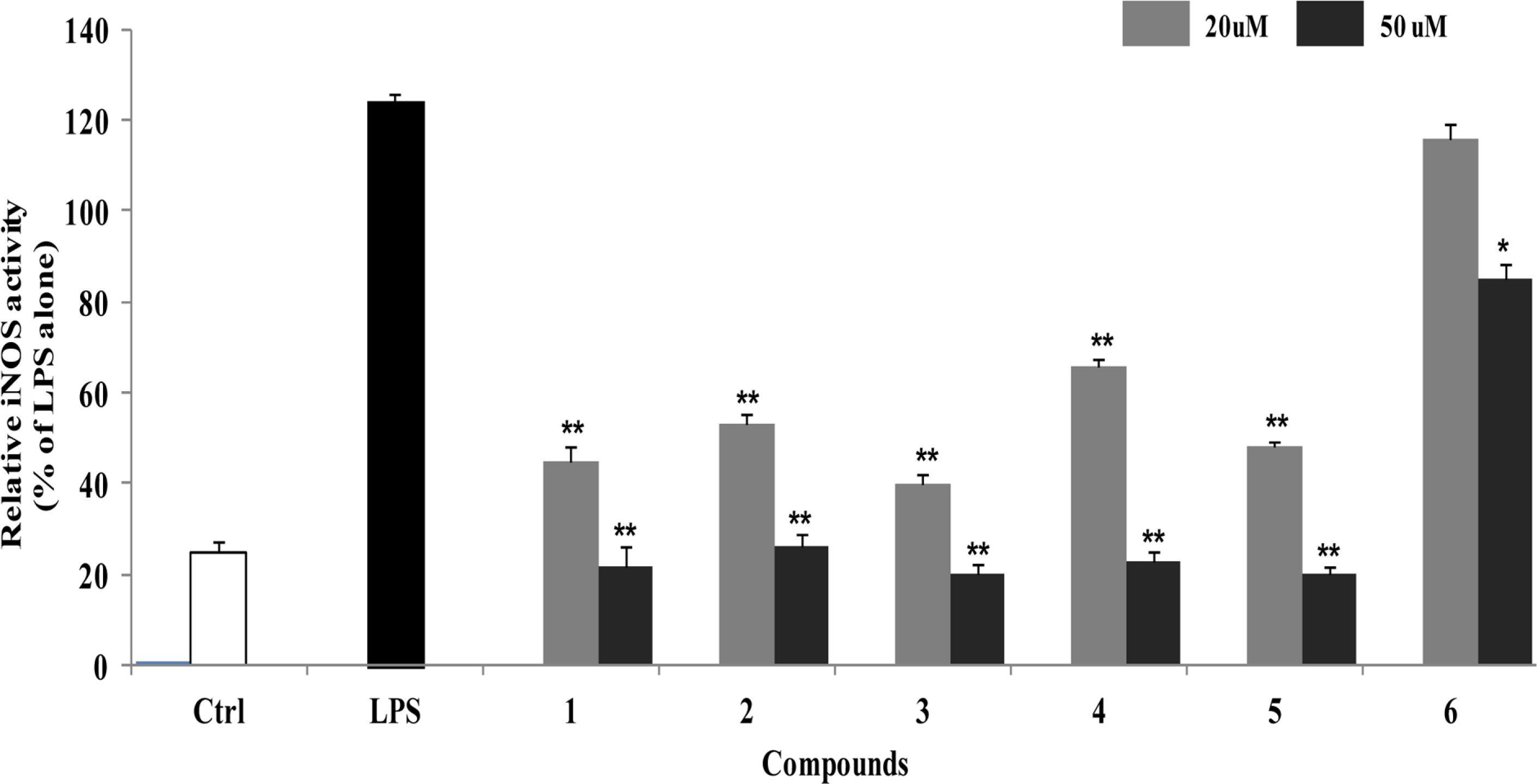
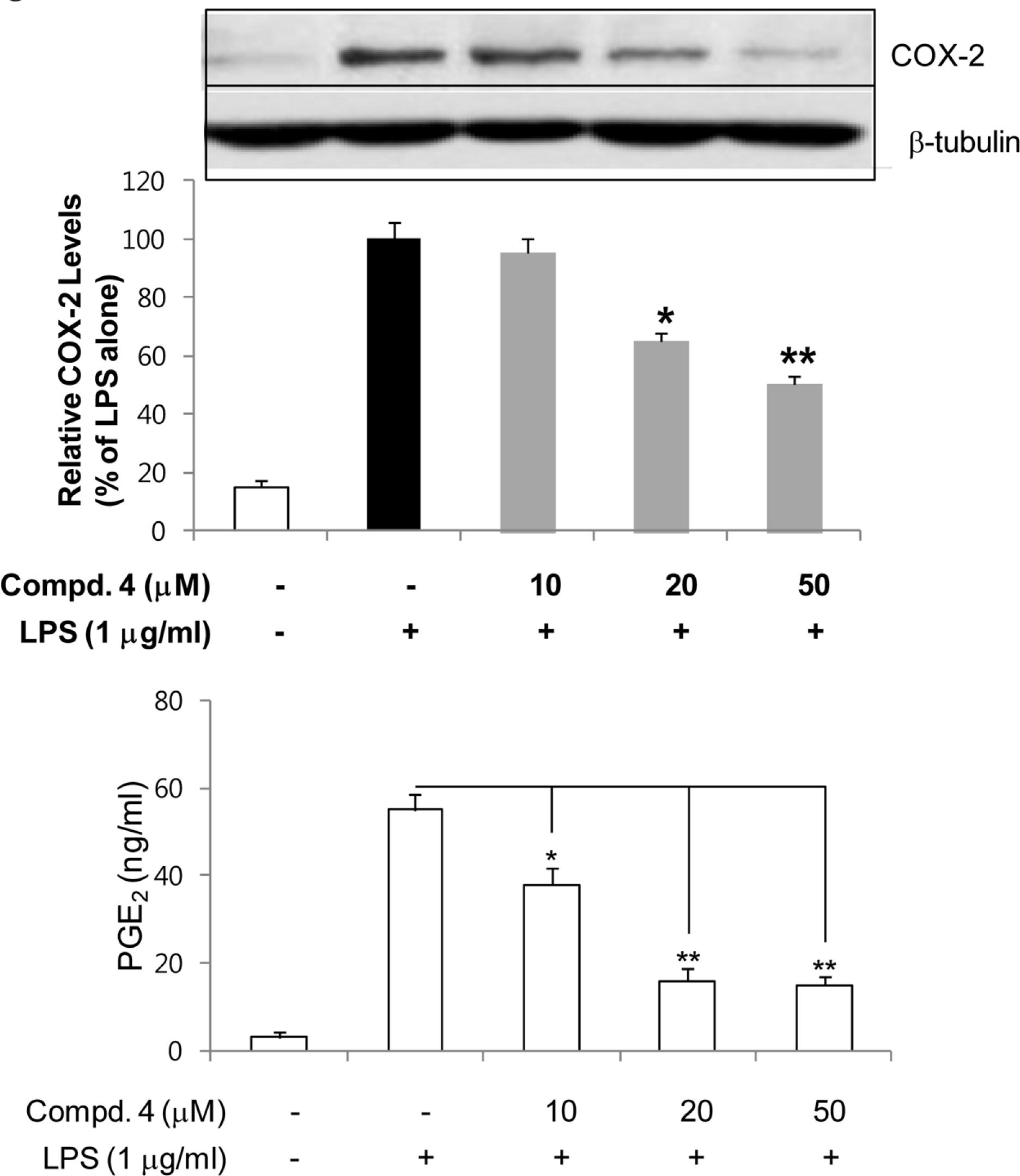
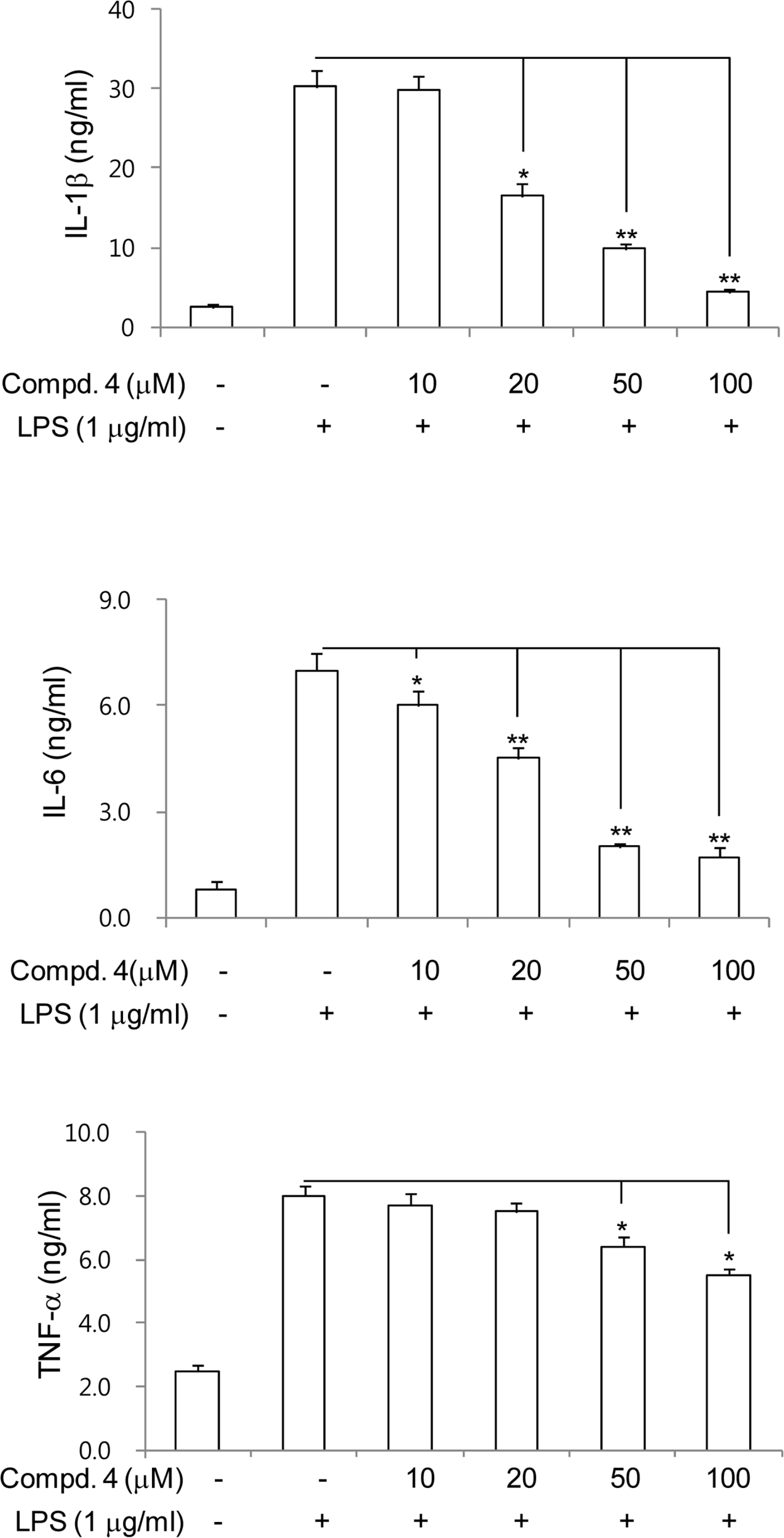
- Anti-inflammatory effects of dihydrobenzofuran neolignans isolated from Euonymus alatus leaves and twigs were evaluated in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells. Six neolignans, (+)- simulanol (1), (+)-dehydrodiconiferyl alcohol (2), (-)-simulanol (3), (-)-dehydrodiconiferyl alcohol (4), (+)-dihydrodehyrodiconiferyl alcohol (5), threo-buddlenol B (6) effectively inhibited the production of nitric oxide (NO) induced by LPS, and the activity of iNOS. (-)-dehydrodiconiferyl alcohol (4), which showed the most potent inhibitory activity, attenuated the activity of iNOS enzyme and also the expression of iNOS and COX-2 proteins. The subsequent production of pro-inflammatory cytokines, interleukin-1β, interleukin-6, tumor necrosis factor-α and prostaglandin E2 were also inhibited by the pretreatment of RAW264.7 cells with (-)-dehydrodiconiferyl alcohol (4). These neolignans are thought to contribute to anti-inflammatory effects of E. alatus, and expected to be potential candidates to prevent/treat inflammation-related diseases.
Keyword
MeSH Terms
Figure
Reference
-
(1). Kaplanski G., Marin V., Montero-Julian F., Mantovani A., Farnarier C.Trends Immunol. 2003; 24:25–29.(2). Adams D. O., Hamilton T. A.Annu. Rev. Immunol. 1984; 2:283–318.
Article(3). Jeong E. J., Yang H., Kim S. H., Kang S. Y., Sung S. H., Kim Y. C.Food Chem. Toxicol. 2011; 49:1394–1398.(4). Jeong E. J., Cho J. H., Sung S. H., Kim S. Y., Kim Y. C.Bioorg. Med. Chem. Lett. 2011; 15:2283–2286.(5). Akihisa T., Yamamoto K., Tamura T., Iida T., Nambara T., Chang F. C.Chem. Pharm. Bull. 1992; 40:789–791.(6). De Fátima Silva G. D., Duarte L. P., Da Silva Paes H. C., De Sousa J. R., Nonato M. C., Portezani P. J., Mascarenhas Y. P. J.Braz. Chem. Soc. 1998; 9:461–464.(7). Liu C. M., Wang H. X., Wei S. L., Gao K. J.Nat. Prod. 2008; 71:789–792.(8). Fang J. M., Lee C. K., Cheng Y. S.Phytochemistry. 1992; 31:3659–3661.(9). Yang Y. P., Cheng M. J., Teng C. M., Chang Y. L., Tsai I. L., Chen I. S.Phytochemistry. 2002; 61:567–572.(10). Meng J., Jiang T., Bhatti H. A., Siddiqui B. S., Dixon S., Kilburn J. D.Org. Biomol. Chem. 2010; 8:107–113.(11). Lourith N., Katayama T., Suzuki T. J.Wood Sci. 2005; 51:370–378.(12). Matsuda S., Kadota S., Tai T., Kikuchi T.Chem. Pharm. Bull. 1984; 32:5066–5069.(13). Dawson V. L., Brahmbhatt H. P., Mong J. A., Dawson T. M.Neuropharmacology. 1994; 33:1425–1430.(14). Korhonen R., Lahti A., Kankaanranta H., Moilanen E.Curr. Drug Targets Inflamm. Allergy. 2005; 4:471–479.(15). Yamashita T., Kawashima S., Ohashi Y., Ozaki M., Ueyama T., Ishida T., Inoue N., Hirata K., Akita H., Yokoyama M.Circulation. 2000; 101:931–937.(16). Penglis P. S., Cleland L. G., Demasi M., Caughey G. E., James M. J. J.Immunol. 2000; 165:1605–1611.(17). Nathan C.FASEB J. 1992; 6:3051–3064.
Article(18). Marletta M. A. J.Biol. Chem. 1993; 268:12231–12234.(19). Duval D. L., Miller D. R., Collier J., Billings R. E.Mol. Pharmacol. 1996; 50:277–284.(20). Son H. J., Lee H. J., Yun-Choi H. S., Ryu J. H.Planta Med. 2000; 66:469–471.(21). Chen T. H., Kao Y. C., Chen B. C., Chen C. H., Chan P., Lee H. M.Eur. J. Pharmacol. 2006; 541:138–146.(22). Hamasaki Y., Kobayashi I., Zaitu M., Tsuji K., Kita M., Hayasaki R., Muro E., Yamamoto S., Matsuo M., Ichimaru T., Miyazaki S.Planta Med. 1999; 65:222–226.(23). Wang J. P., Raung S. L., Chen C. C., Kuo J. S., Teng C. M.Naunyn Schmiedebergs Arch. Pharmacol. 1993; 348:663–669.(24). Oh J. H., Kang L. L., Ban J. O., Kim Y. H., Kim K. H., Han S. B., Hong J. T.Chem. Biol. Interact. 2009; 180:506–514.(25). Choi M. S., Lee S. H., Cho H. S., Kim Y., Yun Y. P., Jung H. Y., Jung J. K., Lee B. C., Pyo H. B., Hong J. T.Eur. J. Pharmacol. 2007; 556:181–189.(26). Connell L., Mclnnes I. B.Best Pract. Res. Clin. Rheumatol. 2006; 20:865–878.(27). Aggarwal B. B., Natarajan K.Eur. Cytokine Netw. 1996; 7:93–124.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Euonymus alatus Extract on Antitumor Activity and Toxicity of Doxorubicin
- Protective effect of euonymus alatus extract on experimental liver injury in mice
- Silymarin Inhibits Morphological Changes in LPS-Stimulated Macrophages by Blocking NF-kappaB Pathway
- Inhibitory activity of Euonymus alatus against alpha-glucosidase in vitro and in vivo
- Effect of Caffeic Acid Phenethyl Ester on Lipopolysaccharide-induced Murine Macrophage Activation