Lab Anim Res.
2014 Sep;30(3):95-103. 10.5625/lar.2014.30.3.95.
Characterization of allergic response induced by repeated dermal exposure of IL-4/Luc/CNS-1 transgenic mice to low dose formaldehyde
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Clinical Laboratory Science, Dong-Eui University, Busan, Korea.
- KMID: 2312120
- DOI: http://doi.org/10.5625/lar.2014.30.3.95
Abstract
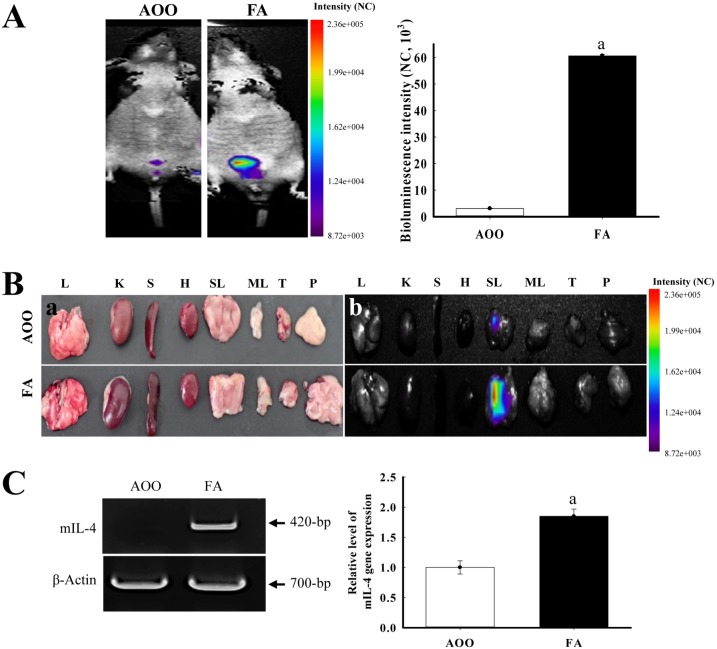
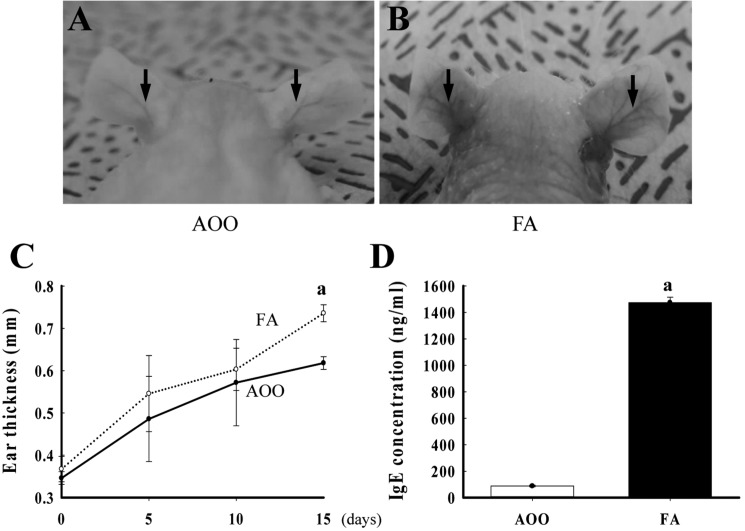
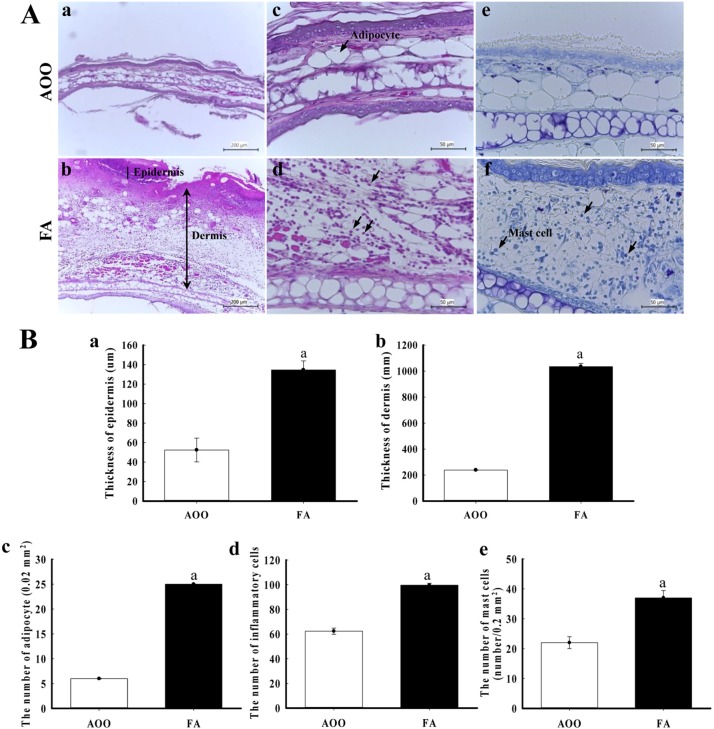
- Although formaldehyde (FA) is known to be a major allergen responsible for allergic contact dermatitis, there are conflicting reports regarding correlation between FA exposure and interleukin (IL-4) expression. To investigate whether allergic responses including IL-4 expression were induced by repeated dermal exposure to low dose FA, alterations in the luciferase signal and allergic phenotypes were measured in IL-4/Luc/CNS-1 transgenic (Tg) mice containing luciferase cDNA under control of the IL-4 promoter after exposure to 4% FA for 2 weeks. High levels of luciferase were detected in the abdominal region of the whole body and submandibular lymph node (SLN) of FA treated mice. Additionally, the ear thickness and IgE concentration were significantly upregulated in the FA treated group when compared with the acetone olive oil (AOO) treated group. FA treated mice showed enhanced auricular lymph node (ALN) weight, epidermis and dermis thickness, and infiltration of inflammatory cells. Furthermore, the expression of IL-6 among T helper 2 cytokines was higher in the FA treated group than the AOO treated group, while vascular endothelial growth factor (VEGF) levels remained constant. Overall, the results presented herein provide additional evidence that various allergic responses may be successfully induced in IL-4/Luc/CNS-1 Tg mice after exposure to low dose FA for 2 weeks. The luciferase signal under the IL-4 promoter may reflect general indicators of the allergic response induced by exposure to low dose FA.
MeSH Terms
-
Acetone
Animals
Cytokines
Dermatitis, Allergic Contact
Dermis
DNA, Complementary
Ear
Epidermis
Formaldehyde*
Immunoglobulin E
Interleukin-4
Interleukin-6
Interleukins
Luciferases
Lymph Nodes
Mice
Mice, Transgenic*
Olea
Phenotype
Vascular Endothelial Growth Factor A
Olive Oil
Acetone
Cytokines
DNA, Complementary
Formaldehyde
Immunoglobulin E
Interleukin-4
Interleukin-6
Interleukins
Luciferases
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Günther R, Walter D, Armin OG, Albrecht H. Formaldehyde. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH;2002.2. Marzulli FN, Maibach HI. The use of graded concentrations in studying skin sensitizers: experimental contact sensitization in man. Food Cosmet Toxicol. 1974; 12(2):219–227. PMID: 4459237.
Article3. Nethercott JR, Holness DL. Contact dermatitis in funeral service workers. Contact Dermatitis. 1988; 18(5):263–267. PMID: 2970932.
Article4. Dearman RJ, Basketter DA, Evans P, Kimber I. Comparison of cytokine secretion profiles provoked in mice by glutaraldehyde and formaldehyde. Clin Exp Allergy. 1999; 29(1):124–132. PMID: 10051711.
Article5. Saneyoshi K, Nohara O, Imai T, Shiraishi F, Moriyama H, Fujimaki H. IL-4 and IL-6 production of bone marrow-derived mast cells is enhanced by treatment with environmental pollutants. Int Arch Allergy Immunol. 1997; 114(3):237–245. PMID: 9363904.
Article6. Xu B, Aoyama K, Takeuchi M, Matsushita T, Takeuchi T. Expression of cytokine mRNAs in mice cutaneously exposed to formaldehyde. Immunol Lett. 2002; 84(1):49–55. PMID: 12161283.
Article7. Saito A, Tanaka H, Usuda H, Shibata T, Higashi S, Yamashita H, Inagaki N, Nagai H. Characterization of skin inflammation induced by repeated exposure of toluene, xylene, and formaldehyde in mice. Environ Toxicol. 2011; 26(3):224–232. PMID: 19904815.
Article8. De Jong WH, Arts JH, De Klerk A, Schijf MA, Ezendam J, Kuper CF, Van Loveren H. Contact and respiratory sensitizers can be identified by cytokine profiles following inhalation exposure. Toxicology. 2009; 261(3):103–111. PMID: 19422874.
Article9. Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, Lee CK, Hwang DY. IL-6, VEGF, KC and RANTES are a major cause of a high irritant dermatitis to phthalic anhydride in C57BL/6 inbred mice. Allergol Int. 2010; 59(4):389–397. PMID: 20864798.
Article10. Lee YJ, Kim JE, Kwak MH, Go J, Kim DS, Son HJ, Hwang DY. Quantitative evaluation of the therapeutic effect of fermented soybean products containing a high concentration of GABA on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. Int J Mol Med. 2014; 33(5):1185–1194. PMID: 24604257.
Article11. Gao XK, Nakamura N, Fuseda K, Tanaka H, Inagaki N, Nagai H. Establishment of allergic dermatitis in NC/Nga mice as a model for severe atopic dermatitis. Biol Pharm Bull. 2004; 27(9):1376–1381. PMID: 15340222.
Article12. Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003; 15(4):399–404. PMID: 12891053.
Article13. Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006; 212:238–255. PMID: 16903918.
Article14. Kapp A. The role of eosinophils in the pathogenesis of atopic dermatitis--eosinophil granule proteins as markers of disease activity. Allergy. 1993; 48(1):1–5. PMID: 8457021.15. Dubois GR, Bruijnzeel PL. IL-4-induced migration of eosinophils in allergic inflammation. Ann N Y Acad Sci. 1994; 725:268–273. PMID: 8030998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation
- IL-13 and STAT6 signaling involve in low dose lipopolysaccharide induced murine model of asthma
- Repeated Ozone Exposure Induces Th2 Immune Responses in Mice
- IL-4-deficient Mice Aggravate Hypersensitivity Pneumonitis
- Role of Mast Cells in a Aspergillus Murine Model of Allergic Rhinitis