Lab Anim Res.
2010 Sep;26(3):293-300. 10.5625/lar.2010.26.3.293.
Dietary Selenium Supplement Prevents Colon Carcinogenesis Induced by Azoxymethane and Dextran Sodium Sulfate in ICR Mice
- Affiliations
-
- 1Department of Veterinary Medicine, College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. beomjun@cbu.ac.kr
- 2Department of Biotechnology and Biomedicine, Chungbuk Province College, Okcheon, Korea.
- KMID: 2312080
- DOI: http://doi.org/10.5625/lar.2010.26.3.293
Abstract
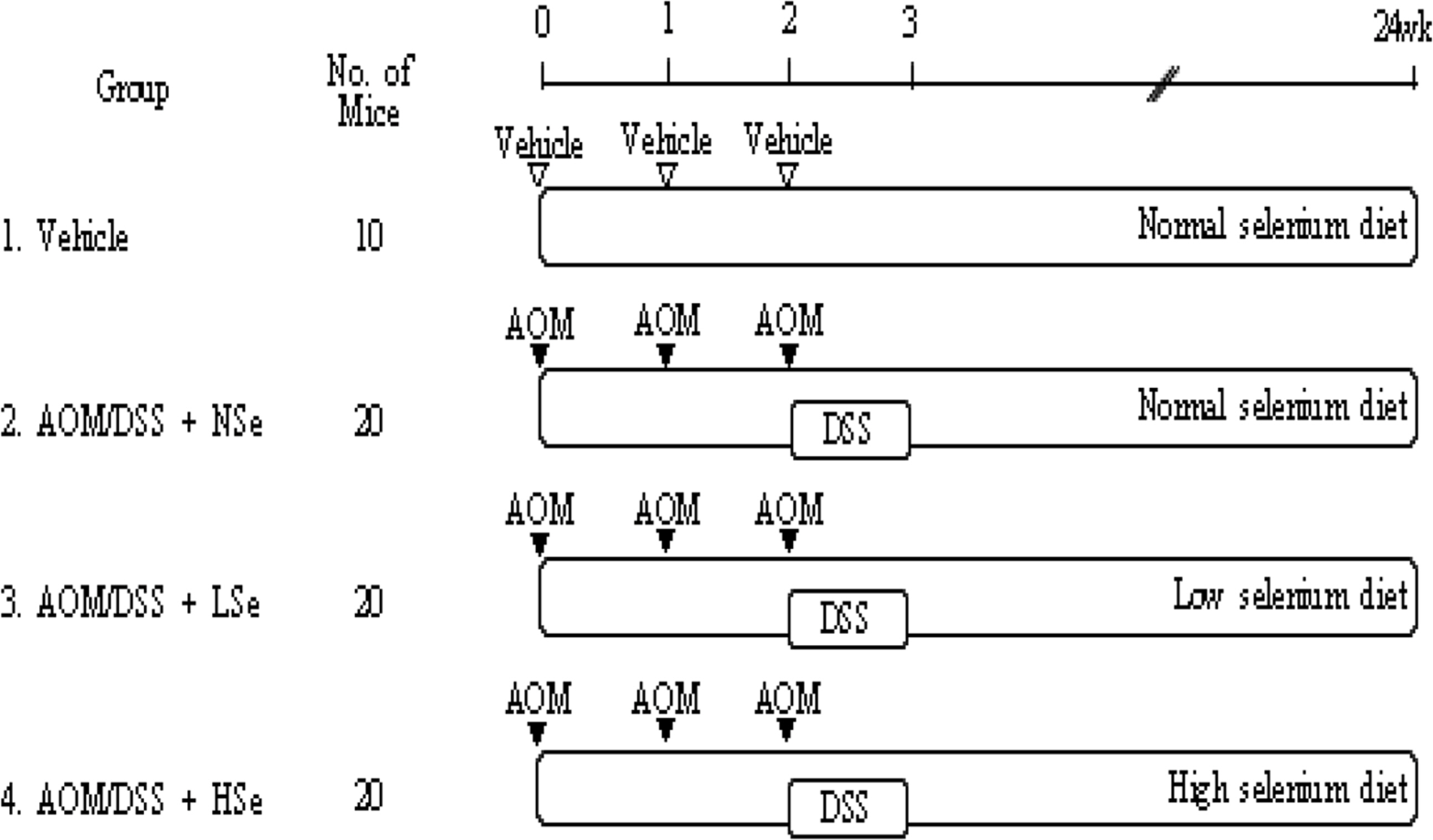
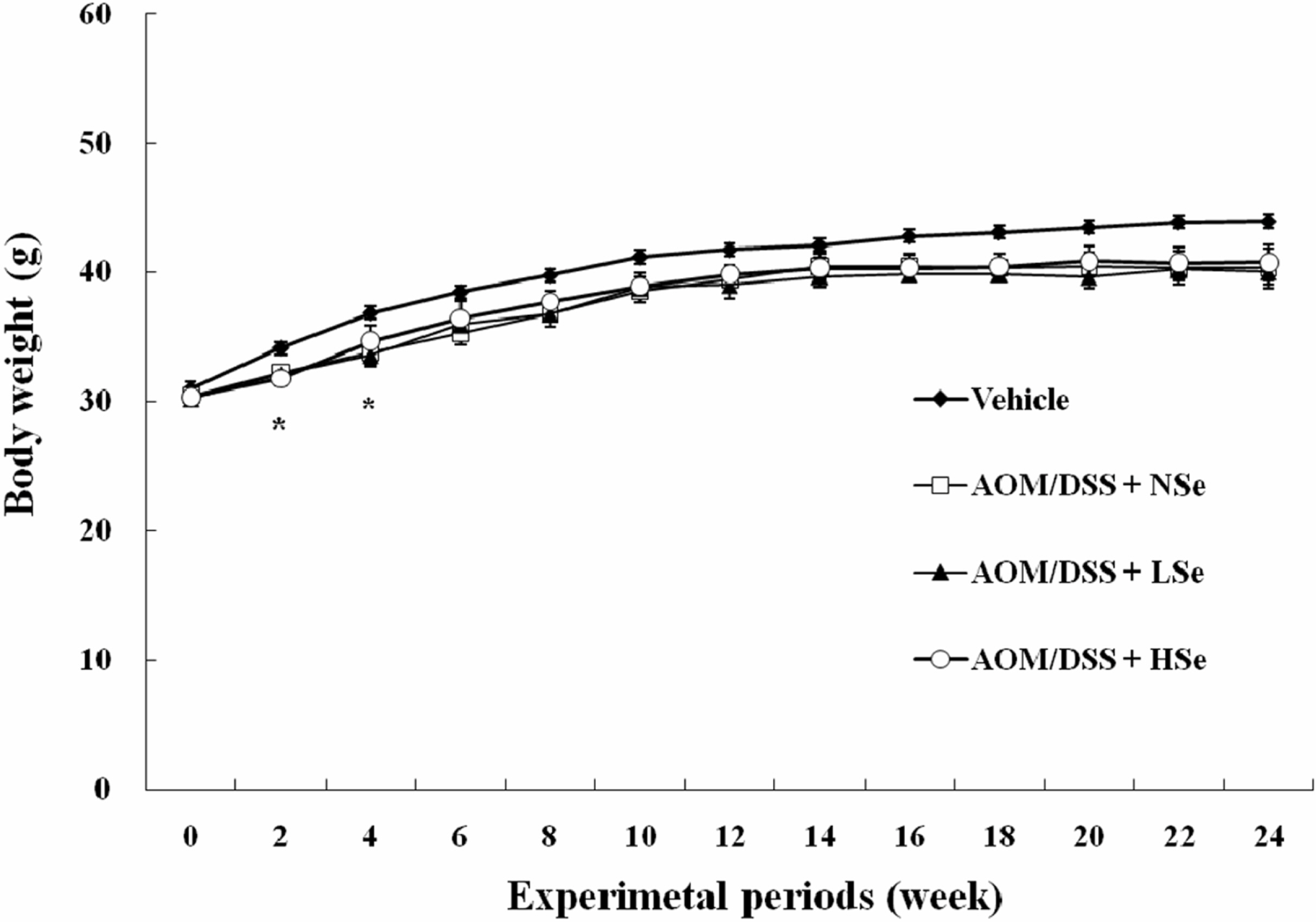
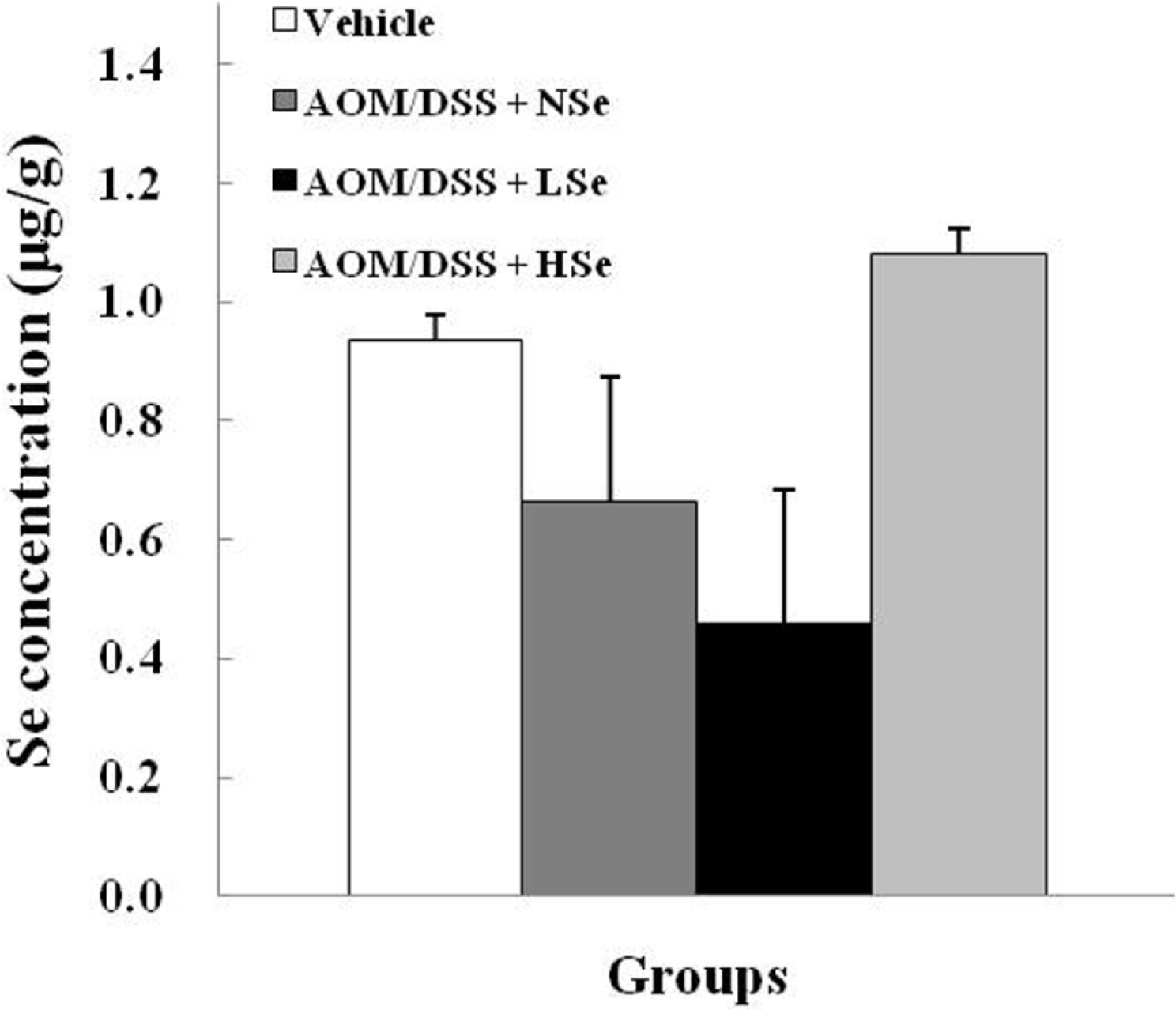
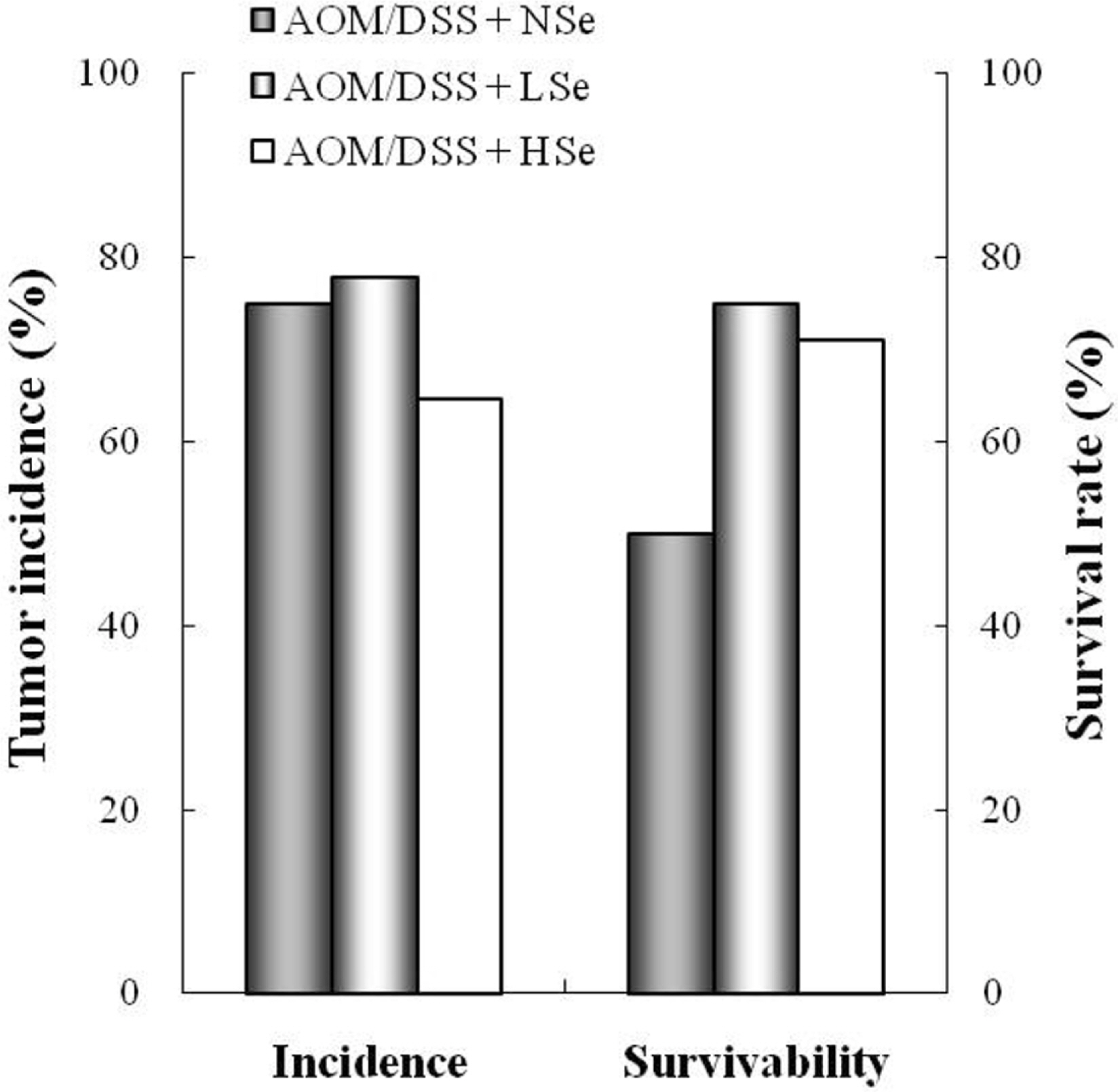
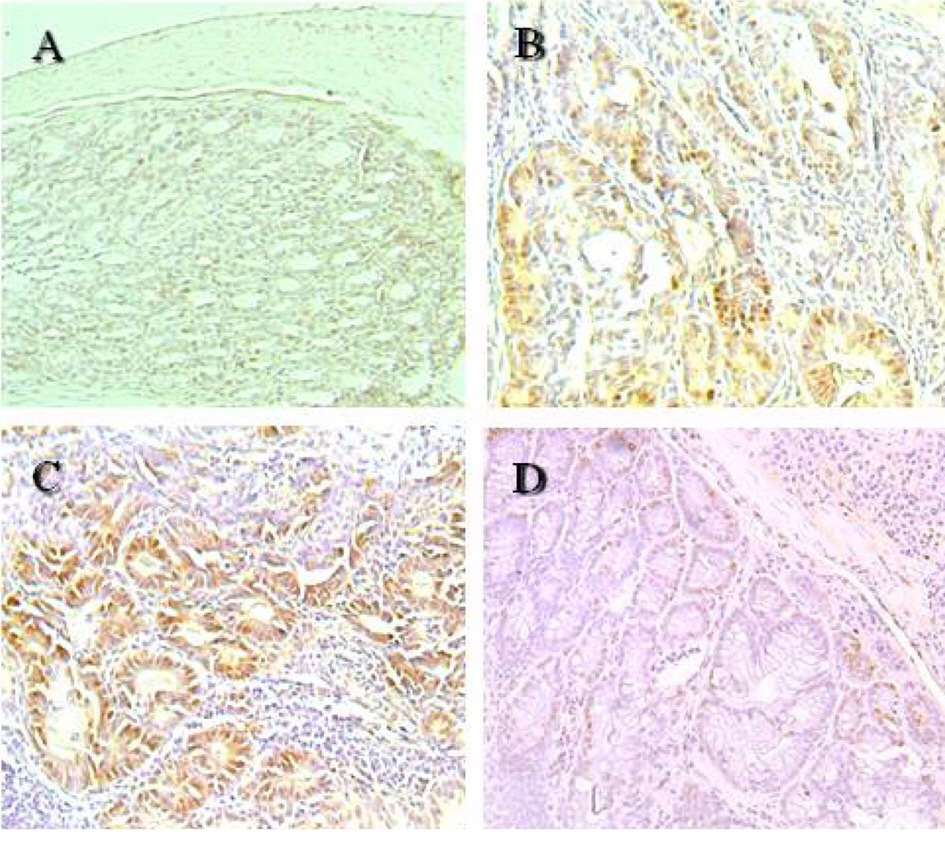
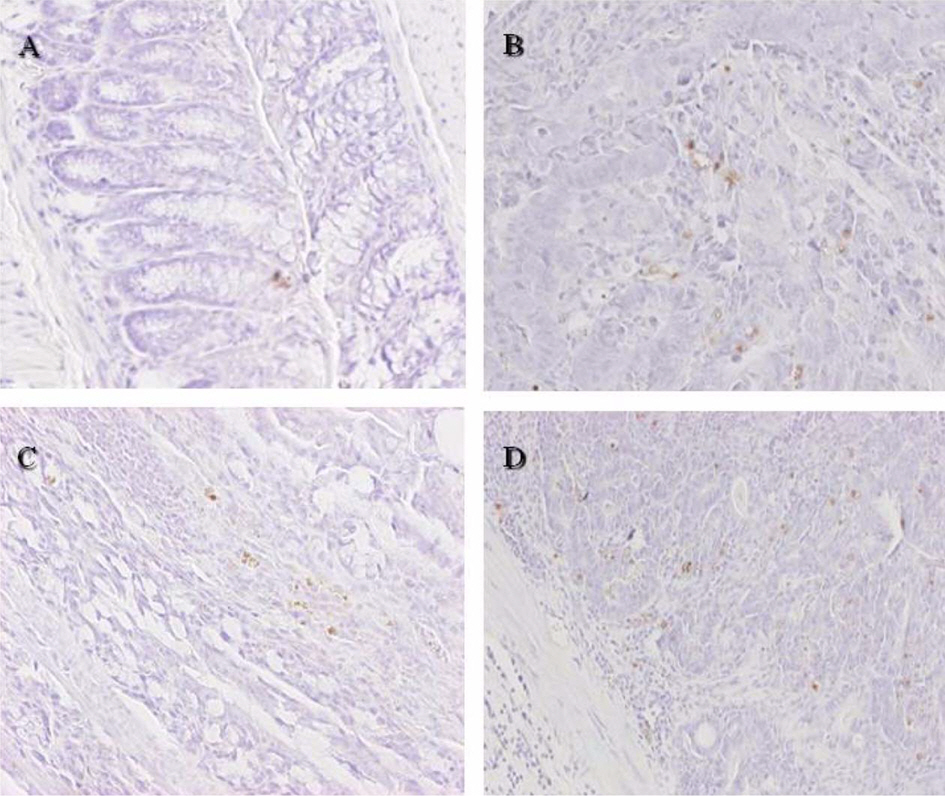
- The role of selenium (Se) in modulating colon carcinogenesis induced by azoxymethane (AOM) followed by dextran sodium sulfate (DSS) was investigated in mice. Five-week old ICR mice were fed on diets containing different concentrations (0.02, 0.1 or 0.5 ppm) of Se for 24 weeks. Animals received three (0-2nd weeks) intraperitoneal injections of AOM (10 mg/kg body weight), followed by 2% DSS with drinking water for additional 1 week. There were 4 experimental groups including vehicle control group, positive control group given AOM/DSS with AIN-93G normal diet containing 0.1% Se (NSe), a low (0.02 ppm)-Se diet group (LSe) and a high (0.5 ppm)-Se diet group (HSe). Hematology was analyzed with a blood cell differential counter. Liver Se was analyzed by inductively coupled plasma-mass spectroscopy. Cell proliferation and apoptosis were determined by using proliferating cell nuclear antigen (PCNA) for proliferative activity and apoptotic index by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), respectively. HSe group showed a low incidence of colonic tumor (64.7%), compared with the NSe positive control (75%) and LSe (77.8%) groups. In contrast, HSe group exhibited lower rate of PCNA-positive cells (39.3+/-6.9%) than positive control (64.3+/-0.3%) and LSe (57.3+/-2.9%) groups. In addition, apoptotic index of HSe group was higher than those of positive control and LSe groups. These results indicate that Se is a chemopreventive agent for colon carcinogenesis induced by AOM+DSS in male ICR mice.
Keyword
MeSH Terms
-
Animals
Apoptosis
Azoxymethane
Blood Cells
Cell Proliferation
Colon
Colonic Neoplasms
Dextrans
Diet
Drinking Water
Hematology
Humans
Incidence
Injections, Intraperitoneal
Liver
Male
Mice
Mice, Inbred ICR
Organothiophosphorus Compounds
Proliferating Cell Nuclear Antigen
Selenium
Sodium
Spectrum Analysis
Sulfates
Azoxymethane
Dextrans
Drinking Water
Organothiophosphorus Compounds
Proliferating Cell Nuclear Antigen
Selenium
Sodium
Sulfates
Figure
Reference
-
Abdulah R.., Miyazaki K.., Nakazawa M.., Koyama H.2005. ).Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 19(2-3):141–150.
ArticleBeno I.., Klvanova J.., Magalova T.., Brtkova A.2000. Blood levels of natural antioxidants in gastric and colorectal precancerous lesions and cancers in Slovakia. Neoplasma. 47(1):37–40.Bravo R.., Frank R.., Blundell P.A.., Macdonald-Bravo H.1987. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 326(6112):515–517.Choi Y.S.., Hesketh J.E.2006. Nutritional Biochemistry of selenium. J. Kor. Soc. Food Sci. Nutr. 35(5):661–670.Clark L.C.., Combs G.F. Jr.., Turnbull B.W.., Slate E.H.., Chalker D.K.., Chow J.., Davis L.S.., Glover R.A.., Graham G.F.., Gross E.G.., Krongrad A.., Lesher J.L. Jr.., Park H.K.., Sanders B.B. Jr.., Smith C.L.., Taylor J.R.1996. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 276(24):1957–1963.
ArticleCombs G.F. Jr.2005. Current evidence and research needs to support a health claim for selenium and cancer prevention. J. Nutr. 135(2):343–347.
ArticleCombs G.F. Jr.., Combs S.B.1984. The nutritional biochemistry of selenium. Annu. Rev. Nutr. 4:257–280.
ArticleDavis C.D.., Johnson W.T.2002. Dietary copper affects azoxymethane-induced intestinal tumor formation and protein kinase C isozyme protein and mRNA expression in colon of rats. J. Nutr. 132(5):1018–1025.
ArticleDavis R. L.., Spallholz J. E.1996. Inhibition of selenite-catalyzed superoxide generation and formation of elemental selenium (Seo) by copper, zinc, and aurintricarboxylic acid (ATA). Biochem. Pharmacol. 51(8):1015–1020.Finley J.W.1999. The retention and distribution by healthy young men of stable isotopes of selenium consumed as selenite, selenate or hydroponically-grown broccoli are dependent on the isotopic form. J. Nutr. 129(4):865–871.
ArticleIp C.., Dong Y.., Ganther H.E.2002. ).New concepts in selenium chemoprevention. Cancer Metastasis Rev. 21(3-4):281–289.Ip C.., Hayes C.., Budnick R.M.., Ganther H.E.1991. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 51(2):595–600.Jemal A.., Murray T.., Ward E.., Samuels A.., Tiwari R.C.., Ghafoor A.., Feuer E.J.., Thun M.J.2005. Cancer statistics, 2005. CA Cancer J. Clin. 55(1):10–30.
ArticleJiang C.., Jiang W.., Ip C.., Ganther H.., Lu J.1999. selenium -induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol. Carcinog. 26(4):213–225.Korea National Statistical Office. 2007. Death and Cause of Death Statistics 2006. KNSO, Daejeon.Milde D.., Novak O.., Stu ka V.., Vyslou il K.., Macha ek J.2001. Serum levels of selenium, manganese, copper, and iron in colorectal cancer patients. Biol. Trace Elem. Res. 79(2):107–114.
ArticleNational Institute of Occupational Safety and Health (NIOSH). 1990. Registry of Toxic Effects of Chemical Substances (RTECS). Canadian Centre for Occupational Health and Safety, Hamilton, Ontario.Okayasu I.., Hatakeyama S.., Yamada M.., Ohkusa T.., Inagaki Y.., Nakaya R.1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 98(3):694–702.
ArticlePatrick L.2004. selenium biochemistry and cancer: a review of the literature. Altern. Med. Rev. 9(3):239–258.Tanaka T.., de Azevedo M.B.., Duran N.., Alderete J.B.., Epifano F.., Genovese S.., Tanaka M.., Curini M.2010. Colorectal cancer chemoprevention by 2 beta-cyclodextrin inclusion compounds of auraptene and 4'-geranyloxyferulic acid. Int. J. Cancer. 126(4):830–840.Tanaka T.., Kohno H.., Suzuki R.., Yamada Y.., Sugie S.., Mori H.2003. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94(11):965–973.
ArticlePawlowicz Z.., Zachara B.A.., Trafikowska U.., Nowicki A.1993. ).Low levels of selenium and activity of glutathione peroxidase in blood of patients with gastrointestinal neoplasms. Pol. Tyg. Lek. 48(25-26):554–556.Rayman M.P.2005. selenium in cancer prevention: a review of the evidence and mechanism of action. Proc. Nutr. Soc. 64(4):527–542.
ArticleRosenberg D.W.., Giardina C.., Tanaka T.2009. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 30(2):183–196.
ArticleRudolf E.., Kralova V.., Cervinka M.2008. selenium and colon cancer–from chemoprevention to new treatment modality. Anticancer Agents Med. Chem. 8(6):598–602.Schrauzer G.N.2000. ).Anticarcinogenic effects of selenium. Cell. Mol. Life Sci. 57(13-14):1864–1873.
ArticleSohn O.S.., Fiala E.S.., Requeijo S.P.., Weisburger J.H.., Gonzalez F.J.2001. Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res. 61(23):8435–8440.Tanaka T.., de Azevedo M.B.., Duran N.., Alderete J.B.., Epifano F.., Genovese S.., Tanaka M.., Curini M.2010. Colorectal cancer chemoprevention by 2 beta-cyclodextrin inclusion compounds of auraptene and 4'-geranyloxyferulic acid. Int. J. Cancer. 126(4):830–840.Tanaka T.., Kohno H.., Suzuki R.., Yamada Y.., Sugie S.., Mori H.2003. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94(11):965–973.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Selenium on Colon Carcinogenesis Induced by Azoxymethane and Dextran Sodium Sulfate in Mouse Model with High-Iron Diet
- Dietary zinc inhibits the formation of colonic preneoplastic lesion induced by azoxymethane and dextran sodium sulfate in mice
- Epithelial Cell-specific Deletion of Microsomal Prostaglandin E Synthase-1 Does Not Influence Colon Tumor Development in Mice
- Increase in dietary protein content exacerbates colonic inflammation and tumorigenesis in azoxymethane-induced mouse colon carcinogenesis
- Suppressive Effect of Zinc on the Formation of Colonic Preneoplastic Lesions in the Mouse Fed High Levels of Dietary Iron