Nutr Res Pract.
2017 Aug;11(4):281-289. 10.4162/nrp.2017.11.4.281.
Increase in dietary protein content exacerbates colonic inflammation and tumorigenesis in azoxymethane-induced mouse colon carcinogenesis
- Affiliations
-
- 1Department of Food Science and Nutrition, Catholic University of Daegu, 13-13, Hayang-ro, Hayang-eup, Gyeongsan, Gyeongbuk 38430, Korea. kimeunj@cu.ac.kr
- KMID: 2385466
- DOI: http://doi.org/10.4162/nrp.2017.11.4.281
Abstract
- BACKGROUND
/OBJECTIVE: The incidence of colorectal cancer (CRC) has been attributed to higher intake of fat and protein. However, reports on the relationship between protein intake and CRC are inconsistent, possibly due to the complexity of diet composition. In this study, we addressed a question whether alteration of protein intake is independently associated with colonic inflammation and colon carcinogenesis.
MATERIALS/METHODS
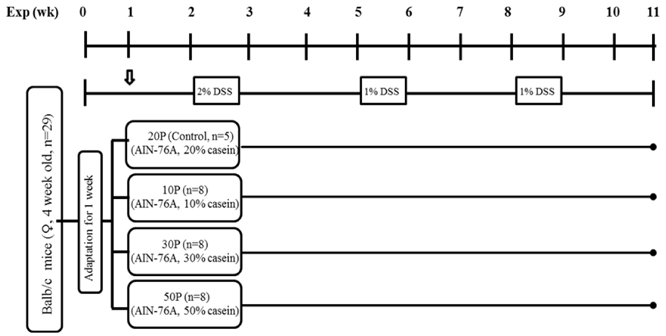
Balb/c mice were randomly divided into 4 experimental groups: 20% protein (control, 20P, 20% casein/kg diet), 10% protein (10P, 10% casein/kg diet), 30% protein (30P, 30% casein/kg diet), and 50% protein (50P, 50% casein/kg diet) diet groups and were subjected to azoxymethane-dextran sodium sulfate induced colon carcinogenesis.
RESULTS
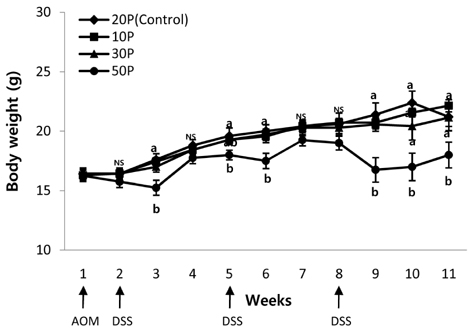
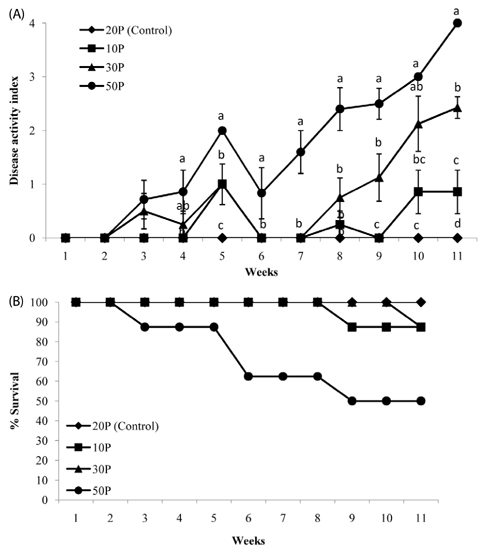
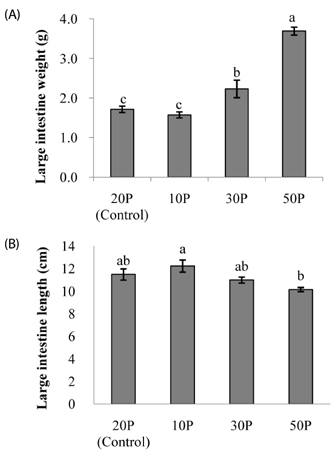
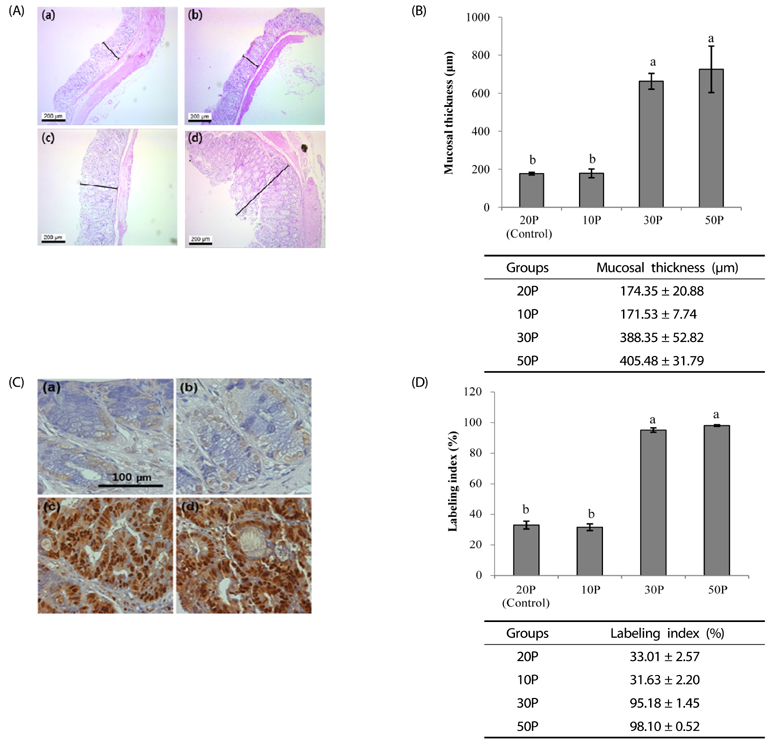
As the protein content of the diet increased, clinical signs of colitis including loss of body weight, rectal bleeding, change in stool consistency, and shortening of the colon were worsened. This was associated with a significant decrease in the survival rate of the mice, an increase in proinflammatory protein expression in the colon, and an increase in mucosal cell proliferation. Further, colon tumor multiplicity was dramatically increased in the 30P (318%) and 50P (438%) groups compared with the control (20P) group.
CONCLUSIONS
These results suggest that a high protein diet stimulates colon tumor formation by increasing colonic inflammation and proliferation.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Dual Effects of High Protein Diet on Mouse Skin and Colonic Inflammation
Xuelei Cui, Eunjung Kim
Clin Nutr Res. 2018;7(1):56-68. doi: 10.7762/cnr.2018.7.1.56.Differential effects of various dietary proteins on dextran sulfate sodium-induced colitis in mice
Eunyeong Ahn, Hyejin Jeong, Eunjung Kim
Nutr Res Pract. 2022;16(6):700-715. doi: 10.4162/nrp.2022.16.6.700.
Reference
-
1. World Cancer Research Fund. American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, D.C.: WCRF/AICR;2007.2. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009; 22:191–197.
Article3. Boyle P, Langman JS. ABC of colorectal cancer: epidemiology. BMJ. 2000; 321:805–808.
Article4. Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975; 15:617–631.
Article5. Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA. 2005; 293:172–182.
Article6. Young GP, Le Leu RK. Preventing cancer: dietary lifestyle or clinical intervention? Asia Pac J Clin Nutr. 2002; 11:Suppl 3. S618–S631.
Article7. Kim E, Coelho D, Blachier F. Review of the association between meat consumption and risk of colorectal cancer. Nutr Res. 2013; 33:983–994.
Article8. Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch attenuates colonic DNA damage induced by higher dietary protein in rats. Nutr Cancer. 2005; 51:45–51.
Article9. Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol Ther. 2006; 5:267–272.
Article10. Toden S, Bird AR, Topping DL, Conlon MA. Differential effects of dietary whey, casein and soya on colonic DNA damage and large bowel SCFA in rats fed diets low and high in resistant starch. Br J Nutr. 2007; 97:535–543.
Article11. Andriamihaja M, Davila AM, Eklou-Lawson M, Petit N, Delpal S, Allek F, Blais A, Delteil C, Tomé D, Blachier F. Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G1030–G1037.
Article12. Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010; 138:2101–2114.
Article13. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001; 48:526–535.
Article14. Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010; 29:3313–3323.
Article15. Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004; 21:491–501.
Article16. Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996; 178:201–206.
Article17. Noguchi M, Hiwatashi N, Liu Z, Toyota T. Secretion imbalance between tumour necrosis factor and its inhibitor in inflammatory bowel disease. Gut. 1998; 43:203–209.
Article18. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444.
Article19. Byun SY, Kim DB, Kim E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr Res. 2015; 35:726–735.
Article20. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69:238–249.21. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990; 98:694–702.
Article22. Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996; 39:87–92.
Article23. Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J Gastroenterol Hepatol. 2002; 17:1078–1083.
Article24. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998; 115:182–205.
Article25. Kwon HS, Oh SM, Kim JK. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2008; 151:165–173.
Article26. Huang YF, Zhou JT, Qu C, Dou YX, Huang QH, Lin ZX, Xian YF, Xie JH, Xie YL, Lai XP, Su ZR. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NFkappaB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J Ethnopharmacol. 2017; 198:389–398.
Article27. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010; 10:181–193.
Article28. Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004; 14:433–439.
Article29. Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G7–17.
Article30. Tatsuta M, Iishi H, Baba M, Taniguchi H. Enhanced induction of colon carcinogenesis by azoxymethane in Wistar rats fed a low-protein diet. Int J Cancer. 1992; 50:108–111.
Article31. Fleming SE, Youngman LD, Ames BN. Intestinal cell proliferation is influenced by intakes of protein and energy, aflatoxin, and whole-body radiation. Nutr Cancer. 1994; 22:11–30.
Article32. Toden S, Bird AR, Topping DL, Conlon MA. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: attenuation by high-amylose maize starch. Carcinogenesis. 2007; 28:2355–2362.
Article33. Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila) . 2011; 4:1903–1911.
Article34. Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012; 56:184–196.
Article35. Liu X, Blouin JM, Santacruz A, Lan A, Andriamihaja M, Wilkanowicz S, Benetti PH, Tomé D, Sanz Y, Blachier F, Davila AM. High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: the increased luminal bulk connection. Am J Physiol Gastrointest Liver Physiol. 2014; 307:G459–G470.
Article36. Lan A, Blais A, Coelho D, Capron J, Maarouf M, Benamouzig R, Lancha AH Jr, Walker F, Tomé D, Blachier F. Dual effects of a high-protein diet on DSS-treated mice during colitis resolution phase. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G624–G633.
Article37. McConnell BB, Yang VW. The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep. 2009; 5:69–74.
Article38. Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004; 23:63–75.
Article39. Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001; 2:149–156.
Article40. Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997; 57:1233–1237.41. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994; 107:1183–1188.
Article42. Cianchi F, Cortesini C, Fantappiè O, Messerini L, Schiavone N, Vannacci A, Nistri S, Sardi I, Baroni G, Marzocca C, Perna F, Mazzanti R, Bechi P, Masini E. Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am J Pathol. 2003; 162:793–801.43. Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002; 62:6323–6328.44. Li G, Yang T, Yan J. Cyclooxygenase-2 increased the angiogenic and metastatic potential of tumor cells. Biochem Biophys Res Commun. 2002; 299:886–890.
Article45. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998; 93:705–716.
Article46. Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004; 555:107–119.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of luteolin on chemical induced colon carcinogenesis in high fat diet-fed obese mouse
- Epithelial Cell-specific Deletion of Microsomal Prostaglandin E Synthase-1 Does Not Influence Colon Tumor Development in Mice
- Dual Effects of High Protein Diet on Mouse Skin and Colonic Inflammation
- Dietary zinc inhibits the formation of colonic preneoplastic lesion induced by azoxymethane and dextran sodium sulfate in mice
- Açaà Berries Inhibit Colon Tumorigenesis in Azoxymethane/Dextran Sulfate Sodium-Treated Mice