Lab Anim Res.
2010 Sep;26(3):233-239. 10.5625/lar.2010.26.3.233.
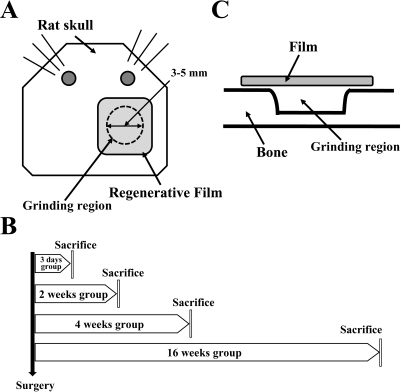
The Bone Grinding and Scaffold Grafting Techniques for Guide Bone Regeneration Induce the Stress on the Rat Brain
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University/PNU-Laboratory Animal Resources Center, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Multidisciplinary Technology Institute, Hoseo University, Asan, Korea.
- KMID: 2312072
- DOI: http://doi.org/10.5625/lar.2010.26.3.233
Abstract
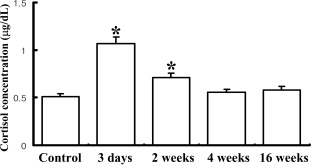
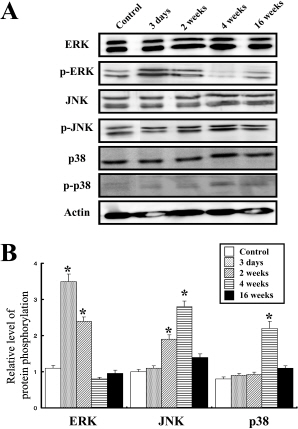
- Guided bone regeneration (GBR) is a technique that a barrier membrane is placed over the bone defect to prevent the cell growth from the connective tissue and epithelium. In this study, in order to determine whether GBR technique could induce stress in rats, the standardized bone defect in rat calvaria was covered with apatitte membrane. Bone and brain tissues were collected from rats at 3 days, 2, 4, and 16 weeks post-operation, and then alteration of the new bone formation at the defects and stress-related factors were detected with histological examination and Western blot, respectively. From 4 to 16 weeks after the operation, the apatitte membrane was attached to the region of regenerated bone and encapsulated with a thick fibrous layer. Furthermore, the concentration of cortisol, a good indicator of stress, significantly increased 3 days post-operation. However, the increase at 3 days was returned to the basal level in 2 weeks. In Western blot analysis, the highest phosphorylation level of extracellular signal-regulated kinase (ERK) was observed 3 day post-operation, while those of the c-jun N-terminal kinase (JNK) and p38 were detected 4 weeks post-operation. Taken together, the results suggest that GBR technique may induce the serious stress on the brain tissue via the induction of ERK phosphorylation during 2 weeks, and that the stress responses restored in 4 week via JNK and p38 signaling pathway.
Keyword
MeSH Terms
Figure
Reference
-
Aguila H.N.., Pakes S.P.., Lai W.C.., Lu Y.S.1988. The effect of transportation stress on splenic natural killer cell activity in C57BL/6 mice. Lab. Anim. Sci. 38(2):148–151.Aslan M.., Simsek G.., Dayi E.2004. Guided bone regeneration (GBR) on healing bone defects: A histological study in rabbits. J. Contemp. Dent. Pract. 5(2):114–123.Bornstein S.R.., Engeland W.C.., Ehrhart-Bornstein M.., Herman J.P.2008. Dissociation of ACTH and glucocorticoids. Trends Endocrinol. Metab. 19(5):175–180.
ArticleChoi J.H.., Kim H.1990. The effects of tryptophan and tyrosine-enriched diet on the serum cortisol, glucose and free fatty acid levels of stressed rats. Korean J. Nutr. 23(4):229–236.Dalin A.M.., Magnusson U.., Haggendal J.., Nyberg L.1993. The effect of transport stress on plasma levels of catecholamine, cortisol, corticosteriod-binding globulin, blood cell count, and lymphocyte proliferation in pigs. Acta Vet. Scand. 34(1):59–68.Dallmann R.., Steinlechner S.., von Horsten S.., Karl T.2006. Stress-induced hyperthermia in the rat: comparison of classical and novel recording methods. Lab. Anim. 40:186–193.
ArticleDe Kloet E.R.., Sutanto W.., Rots N.., van Haarst A.., van den Berg D.., Oitzl M.., van Eekelen A.., Voorhuis D.1991. Plasticity and function of brain corticosteroid receptors during aging. Acta. Endocrinol. (Copenh.) 125 Suppl. 1:65–72.Diamond D.M.1992. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 2(4):431–430.
Article431-430. Fanger G.R.., Gerwins P.., Widmann C.., Jarpe M.B.., Johnson G.L.1997. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr. Opin. Genet. Dev. 7(1):67–74.Hains B.C.., Waxman S.G.2006. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 26:4308–4317.
ArticleHench L.L.., Wilson J.1999. An Introduction to Bioceramics, 2nd ed., World Scientific Publishing Co., Pte. Ltd., Singapore.Huot J.., Houle F.., Marceau F.., Landry J.1997. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 80(3):383–392.
ArticleJi R.R.., Gereau I.V.R.W.., Malcangioc M.., Strichartz G.R.2009. MAP kinase and pain. Brain Res. Rev. 60(1):135–148.
ArticleJin S.X.., Zhuang Z.Y.., Woolf C.J.., Ji R.R.2003. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 23:4017–4022.
ArticleKojima T.., Amizuka N.., Suzuki A.., Henrique P.., de Freitas L.., Yoshizawa M.., Kudo A.., Saito C.., Maeda T.2007. Histological examination of bone regeneration achived by combing grafting with hydroxyapatite and thermoplastic bioresorbable plates. J. Bone Miner. Metab. 25:361–373.Kuhn G.., Lichtwald K.., Hardegg W.., Abel H.1991. The effect of transportation stress in circulating corticosteroids, enzyme activities and hematological values in laboratory dogs. J. Exp. Anim. Sci. 34(3):99–104.Lee S.G.., Hur J.H.., Yuk J.Y.., Kang C.B.2005. Changes of Serum Cortisol Concentrations by Clipping Stress in Dogs. J.Marshall C.J.1994. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4:82–89.
ArticleMoberg G.P.1985. Biological response to stress: key to assessment of animal well-being. In Animal Stress (Moberg, G.P. ed.), pp. 27-49, American Physiology Society, Maryland.Mundell R.D.., Mooney M.P.., Siegel M.I.1993. Osseous guided tissue regeneration using a collagen barier membrane. J. Oral Maxillofac. Surg. 51(9):1004–1012.Nyman R.., Magnusson M.., Sennerby L.., Nyman S.., Lundgren D.1995. Membrane-guided bone regeneration. Acta. Orthop. Scand. 66(2):169–173.Rhee S.J.., Park G.Y.., Kim M.J.., Kim S.O.., Choi J.H.., Chai Y.M.., Hong H.J.., Yun J.Y.1997. Changes of insulin, cortisol, and methallothionein contents according to time in streptozotocin induced diabetic rats. HSJAS 6-1. 93–98.Sandberg E.., Dahlin C.., Linde A.1993. Bone regeneration by the osteopromotion technique using bioabsorbable membranes: an experimental study in rats. J. Oral Maxillofac. Surg. 51(10):1106–1114.
ArticleSanhouri A.A.., Jones R.S.., Dobson H.1989. The effect of different types of transportation on plasma cortisol and testosterone concnetrations in male goats. Br. Vet. J. 145:446–450.Selye H.1976. The Stress of Life, 1st ed., pp. 3-8, McGraw-Hill Book Co., New York.Shim S.B.., Lee S.H.., Kim C.K.., Kim B.G.., Jee S.W.., Lee S.H.., Sin J.S.., Bae C.J.., Woo J.M.., Cho J.S.., Lee E.P.., Choi H.W.., Kim H.S.., Lee J.H.., Jung Y.J.., Cho B.W.., Chae K.R.., Hwang D.Y.2009. Effects of air transportation cause physiological and biochemical changes indicative of stress leading to regulation of chaperone expression levels and corticosterone concentration. Exp. Anim. 58(1):11–17.
ArticleVan Loo P.L.P.., Van der Meer E.., Kruitwagen C.L.J.J.., Koojhaas J.M.., Van Zutphen L.F.M.., Baumans V.2004. Longterm effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab. Anim. 38:169–177.Verheij M.., Bose R.., Lin X.H.., Yao B.., Jarvis W.D.., Grant S.., Birrer M.J.., Szabo E.., Zon L.I.., Kyriakis J.M.., Haimovitz-Friedman A.., Fuks Z.., Kolesnick R.N.1996. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 380:75–79.
ArticleWaskiewicz A.J.., Cooper J.A.1995. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 7(6):798–805.
ArticleZhuang Z.Y.., Wen Y.R.., Zhang D.R.., Borsello T.., Bonny C.., Strichartz G.R.., Decosterd I.., Ji R.R.2006. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 26:3551–3560.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The optimal scaffold for silk sericin‑based bone graft: collagen versus gelatin
- BONE REGENERATION WITH INJECTABLE MPEG-PCL DIBLOCK COPOLYMER AND BONE MARROW MESENCHYMAL STEM CELL
- Bone tissue engineering using PLLA/HA composite scaffold and bone marrow mesenchymal stem cell
- Soft-tissue management for primary closure in immediate implant placement
- A Review and Description of Acetabular Impaction Bone Grafting: Updating the Traditional Technique