Anat Cell Biol.
2016 Jun;49(2):107-115. 10.5115/acb.2016.49.2.107.
5-Aza-2'-deoxycytidine acts as a modulator of chondrocyte hypertrophy and maturation in chick caudal region chondrocytes in culture
- Affiliations
-
- 1Department of Biochemistry, College of Science, King Saud University, Riyadh, Saudi Arabia. shaq@ksu.edu.sa
- KMID: 2308917
- DOI: http://doi.org/10.5115/acb.2016.49.2.107
Abstract
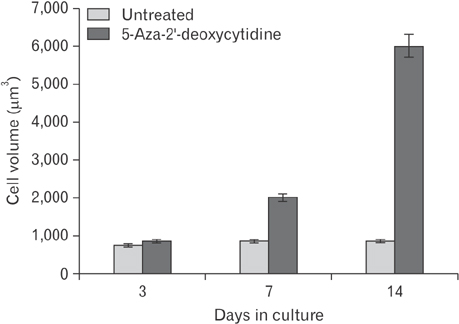
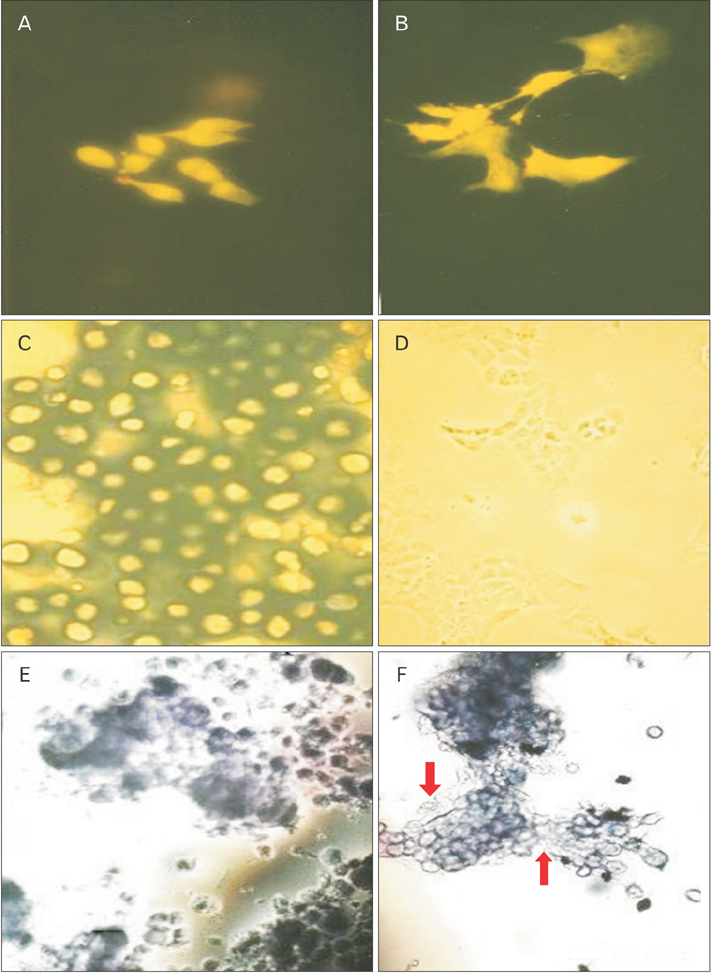
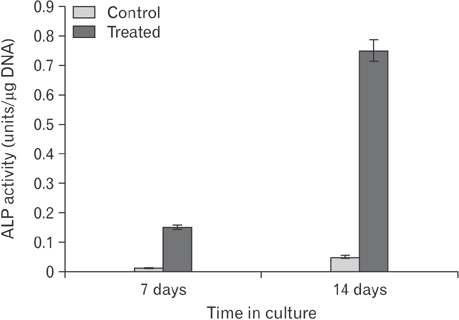
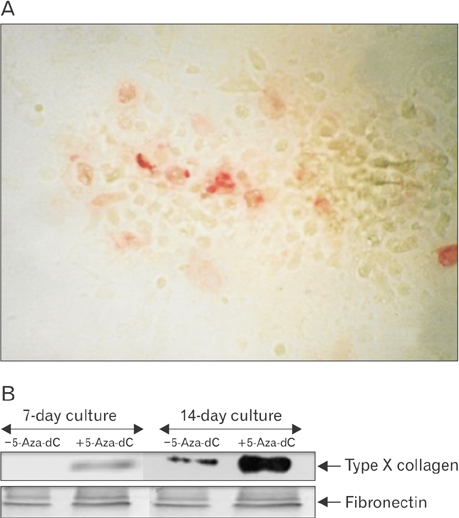
- This study was carried out to explore the effect of DNA hypomethylation on chondrocytes phenotype, in particular the effect on chondrocyte hypertrophy, maturation, and apoptosis. Chondrocytes derived from caudal region of day 17 embryonic chick sterna were pretreated with hypomethylating drug 5-aza-2'-deoxycytidine for 48 hours and then maintained in the normal culture medium for up to 14 days. Histological studies showed distinct morphological changes occurred in the pretreated cultures when compared to the control cultures. The pretreated chondrocytes after 7 days in culture became bigger in size and acquired more flattened fibroblastic phenotype as well as a loss of cartilage specific extracellular matrix. Scanning electron microscopy at day 7 showed chondrocytes to have increased in cell volume and at day 14 in culture the extracellular matrix of the pretreated cultures showed regular fibrillar structure heavily embedded with matrix vesicles, which is the characteristic feature of chondrocyte hypertrophy. Transmission electron microscopic studies indicated the terminal fate of the hypertrophic cells in culture. The pretreated chondrocytes grown for 14 days in culture showed two types of cells: dark cells which had condense chromatin in dark patches and dark cytoplasm. The other light chondrocytes appeared to be heavily loaded with endoplasmic reticulum indicative of very active protein and secretory activity; their cytoplasm had large vacuoles and disintegrating cytoplasm. The biosynthetic profile showed that the pretreated cultures were actively synthesizing and secreting type X collagen and alkaline phosphatase as a major biosynthetic product.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Apoptosis
Cartilage
Cell Size
Chondrocytes*
Chromatin
Collagen Type X
Cytoplasm
DNA
Endoplasmic Reticulum
Endoplasmic Reticulum, Rough
Extracellular Matrix
Fibroblasts
Hypertrophy*
Microscopy, Electron, Scanning
Microscopy, Electron, Transmission
Phenotype
Vacuoles
Alkaline Phosphatase
Chromatin
Collagen Type X
DNA
Figure
Reference
-
1. Marino R. Growth plate biology: new insights. Curr Opin Endocrinol Diabetes Obes. 2011; 18:9–13.2. Chen KS, Tatarczuch L, Mirams M, Ahmed YA, Pagel CN, Mackie EJ. Periostin expression distinguishes between light and dark hypertrophic chondrocytes. Int J Biochem Cell Biol. 2010; 42:880–889.3. Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011; 211:109–121.4. McDevitt CA. Biochemistry of articular cartilage. Nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann Rheum Dis. 1973; 32:364–378.5. Bruckner P, Hörler I, Mendler M, Houze Y, Winterhalter KH, Eich-Bender SG, Spycher MA. Induction and prevention of chondrocyte hypertrophy in culture. J Cell Biol. 1989; 109:2537–2545.6. Wu LN, Genge BR, Wuthier RE. Association between proteoglycans and matrix vesicles in the extracellular matrix of growth plate cartilage. J Biol Chem. 1991; 266:1187–1194.7. Bianco P, Cancedda FD, Riminucci M, Cancedda R. Bone formation via cartilage models: the "borderline" chondrocyte. Matrix Biol. 1998; 17:185–192.8. Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995; 131:483–494.9. Adams CS, Shapiro IM. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit Rev Oral Biol Med. 2002; 13:465–473.10. Horvitz HR, Herskowitz I. Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell. 1992; 68:237–255.11. Christman JK. 5-Azacytidine and 5-aza-2'-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002; 21:5483–5495.12. Brank AS, Eritja R, Garcia RG, Marquez VE, Christman JK. Inhibition of HhaI DNA (Cytosine-C5) methyltransferase by oligodeoxyribonucleotides containing 5-aza-2'-deoxycytidine: examination of the intertwined roles of co-factor, target, transition state structure and enzyme conformation. J Mol Biol. 2002; 323:53–67.13. Zhou GS, Zhang XL, Wu JP, Zhang RP, Xiang LX, Dai LC, Shao JZ. 5-Azacytidine facilitates osteogenic gene expression and differentiation of mesenchymal stem cells by alteration in DNA methylation. Cytotechnology. 2009; 60:11.14. Moazedi-Fuerst FC, Hofner M, Gruber G, Weinhaeusel A, Stradner MH, Angerer H, Peischler D, Lohberger B, Glehr M, Leithner A, Sonntagbauer M, Graninger WB. Epigenetic differences in human cartilage between mild and severe OA. J Orthop Res. 2014; 32:1636–1645.15. Rushton MD, Reynard LN, Barter MJ, Refaie R, Rankin KS, Young DA, Loughlin J. Characterization of the cartilage DNA methylome in knee and hip osteoarthritis. Arthritis Rheumatol. 2014; 66:2450–2460.16. D'Angelo M, Pacifici M. Articular chondrocytes produce factors that inhibit maturation of sternal chondrocytes in serum-free agarose cultures: a TGF-beta independent process. J Bone Miner Res. 1997; 12:1368–1377.17. Von Bertalanffy L, Masin M, Masin F. A new and rapid method for diagnosis of vaginal and cervical cancer by fluorescence microscopy. Cancer. 1958; 11:873–887.18. Steven FS, Suresh U, Wong TL, Griffin MM. The role of inhibitors in the fluorescent staining of benign naevus and malignant melanoma cells with 9-amino acridine and acridine orange. J Enzyme Inhib. 1987; 1:275–287.19. Pashley DH, Tao L, Boyd L, King GE, Horner JA. Scanning electron microscopy of the substructure of smear layers in human dentine. Arch Oral Biol. 1988; 33:265–270.20. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.21. Cheung JO, Hillarby MC, Ayad S, Hoyland JA, Jones CJ, Denton J, Thomas JT, Wallis GA, Grant ME. A novel cell culture model of chondrocyte differentiation during mammalian endochondral ossification. J Bone Miner Res. 2001; 16:309–318.22. Buckwalter JA, Mower D, Ungar R, Schaeffer J, Ginsberg B. Morphometric analysis of chondrocyte hypertrophy. J Bone Joint Surg Am. 1986; 68:243–255.23. Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 1987; 5:509–522.24. D'Angelo M, Billings PC, Pacifici M, Leboy PS, Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP): a role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J Biol Chem. 2001; 276:11347–11353.25. Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 2009; 1790:1592–1598.26. Roach HI, Clarke NM. "Cell paralysis" as an intermediate stage in the programmed cell death of epiphyseal chondrocytes during development. J Bone Miner Res. 1999; 14:1367–1378.27. Roach HI, Clarke NM. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J Bone Joint Surg Br. 2000; 82:601–613.28. Younesi E, Bayati V, Hashemitabar M, Azandeh SS, Bijannejad D, Bahreini A. Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum? Anat Cell Biol. 2015; 48:170–176.29. Blanco FJ, Rego-Pérez I. Editorial: Is it time for epigenetics in osteoarthritis? Arthritis Rheumatol. 2014; 66:2324–2327.30. Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. 2012; 20:339–349.31. Chen T, Ueda Y, Dodge JE, Wang Z, Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol Cell Biol. 2003; 23:5594–5605.32. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002; 16:6–21.33. Pound JC, Green DW, Roach HI, Mann S, Oreffo RO. An ex vivo model for chondrogenesis and osteogenesis. Biomaterials. 2007; 28:2839–2849.34. Gibson G, Lin DL, Roque M. Apoptosis of terminally differentiated chondrocytes in culture. Exp Cell Res. 1997; 233:372–382.35. Cheung JO, Grant ME, Jones CJ, Hoyland JA, Freemont AJ, Hillarby MC. Apoptosis of terminal hypertrophic chondrocytes in an in vitro model of endochondral ossification. J Pathol. 2003; 201:496–503.36. Ahmed YA, Tatarczuch L, Pagel CN, Davies HM, Mirams M, Mackie EJ. Physiological death of hypertrophic chondrocytes. Osteoarthritis Cartilage. 2007; 15:575–586.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Fibroblast Growth Factor Receptors at Different Stages of Differentiation in Chick Embryo Chondrocytes
- Regulation of Cartilage Development and Diseases by Transcription Factors
- The Effects of bFGF, VEGF and Micromass Culture on Proliferation and Differentiation of Human Chondrocytes
- Repair of Osteochondral Defect Using Grafts of Cultured Chondrocytes in Rabbits
- Evaluation of Medium Preparations for Temporomandibular Joint Disc Chondrocytes