Allergy Asthma Immunol Res.
2016 Sep;8(5):445-456. 10.4168/aair.2016.8.5.445.
Synergistic Effect of Dermatophagoides farinae and Lipopolysaccharides in Human Middle ear Epithelial Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chosun University College of Medicine, Gwangju, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Graduate school of Medicine, Seoul National University, Seoul, Korea.
- 3Sensory Organ Research Center, Seoul National University Biomedical Research Institute, Seoul, Korea.
- 4Institute of Allergy and Clinical Immunology, Seoul National University Biomedical Research Institute, Seoul, Korea.
- 5Graduate School of Immunology, Seoul National University, Seoul, Korea.
- 6Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 7Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University College of Medicine, Seoul, Korea. dongkim@snu.ac.kr
- KMID: 2295154
- DOI: http://doi.org/10.4168/aair.2016.8.5.445
Abstract
- PURPOSE
Although the concept of "one airway, one disease," which includes the middle ear space as part of the united airway is well recognized, the role of allergens in otitis media with effusion (OME) is not clearly understood. We aimed to investigate the effect of the interaction between Dermatophagoides farinae (Der f) and lipopolysaccharide (LPS) on the induction of epithelial inflammatory response in vitro.
METHODS
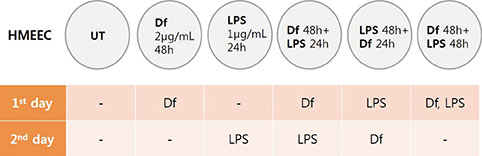
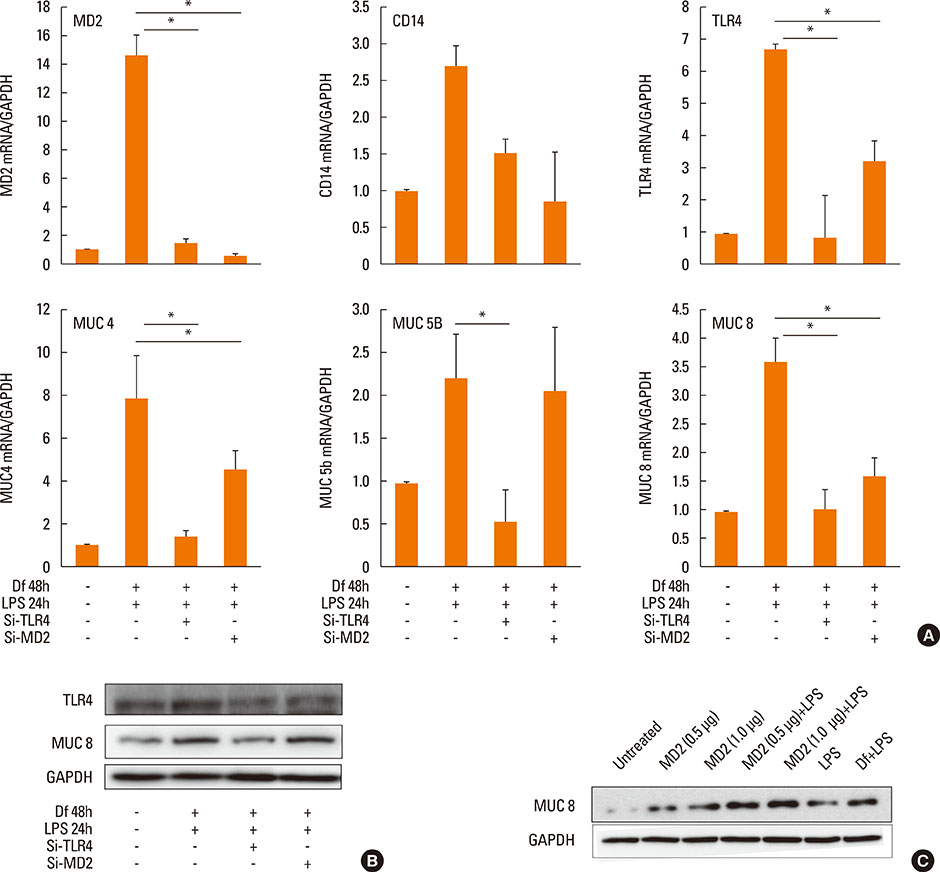
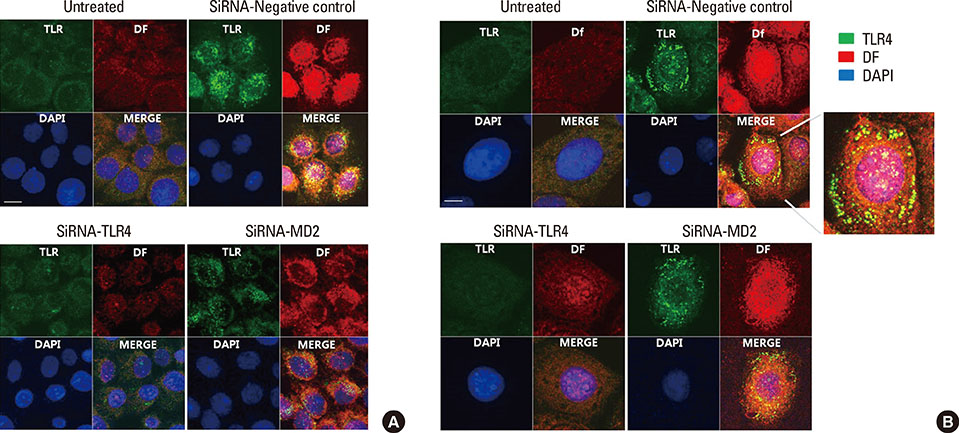
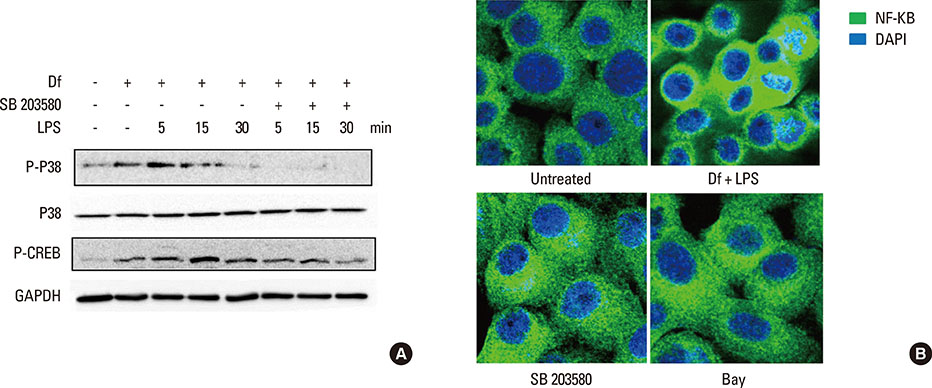
Primary human middle ear epithelial cells were exposed to Der f, LPS, or both in different sequences, and the magnitude of the immunologic responses was compared. The mRNA expressiona of mucin (MUC) 4, 5AC, 5B, 8, GM-CSF, TNF-α, TLR4, and MD-2 were evaluated using real-time PCR. MUC levels before and after siRNA-mediated knockout of TLR4 and MD-2 were assessed. Lastly, the involved cell signaling pathway was evaluated.
RESULTS
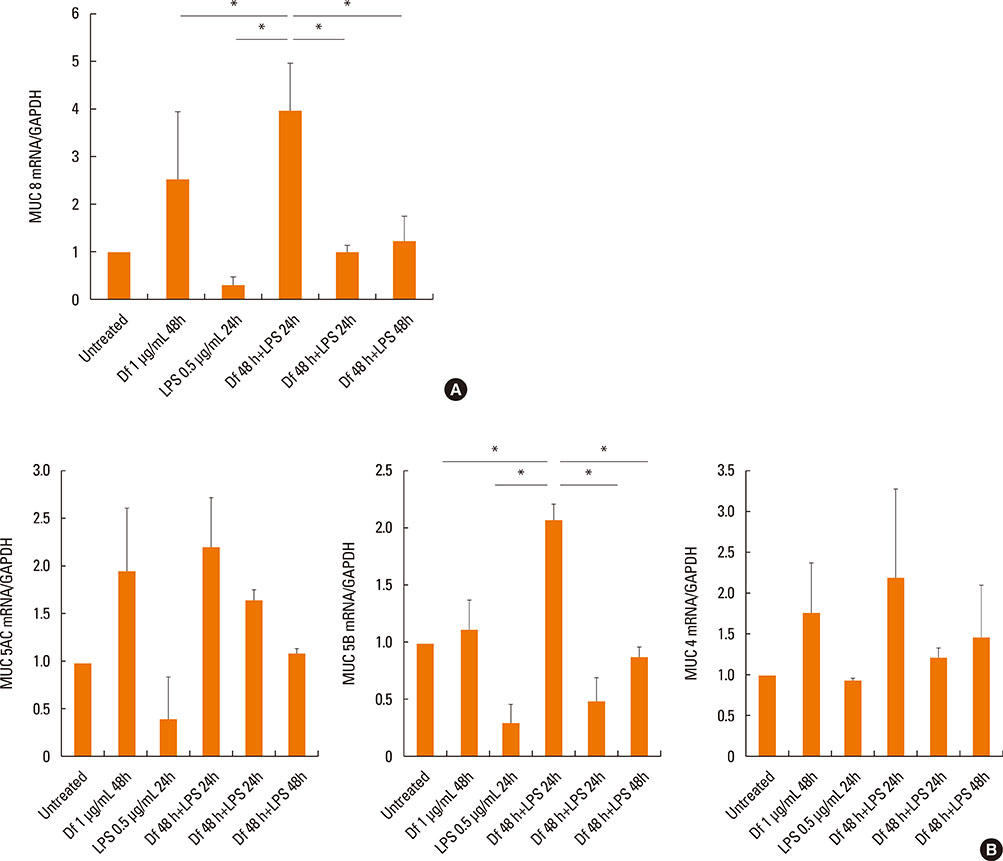
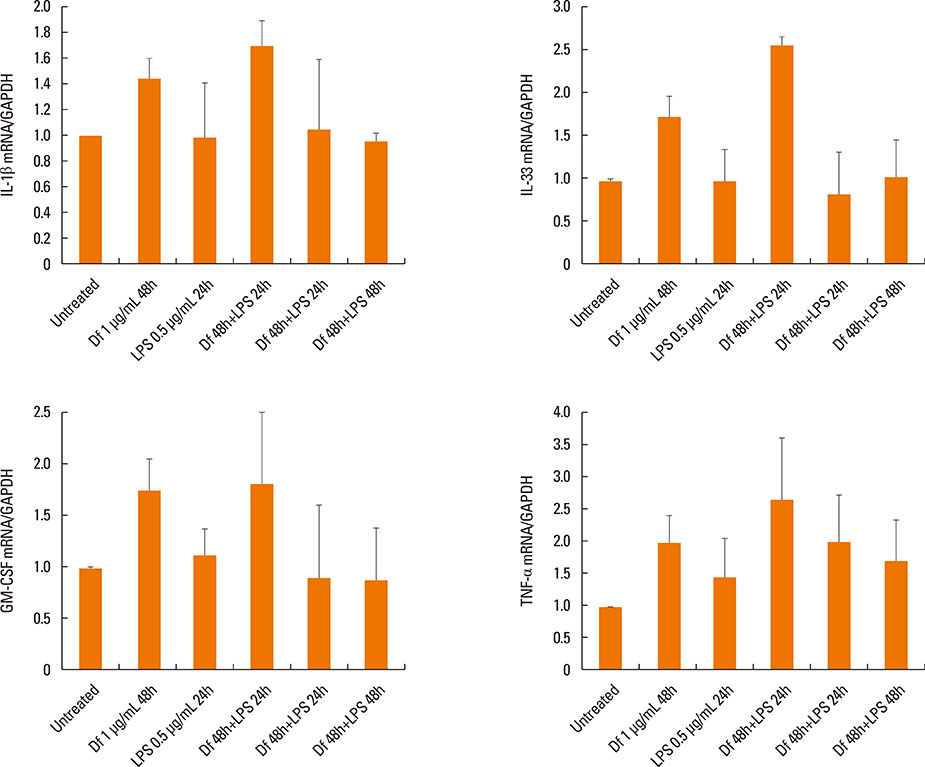
The expressiona of cytokines, and the MUC 4, 5AC, 5B, and 8 genes were augmented by pretreatment with Der f followed by LPS; however, reverse treatment or combined treatment did not induce the same magnitude of response. Increased MUC expression was decreased by TLR4 knockdown, but not by MD-2 knockdown. The signal intensity of MUC 8 was higher in MD-2 over-expressed cells than in those exposed to LPS only. The translocation of nuclear factor-κB was observed in cells pretreated with Der f followed by LPS.
CONCLUSIONS
When Der f treatment preceded LPS exposure, Der f and LPS acted synergistically in the induction of pro-inflammatory cytokines and the MUC gene, suggesting an important role in the development of OME in patients with concealed allergy airway sensitization.
MeSH Terms
-
Allergens
Cytokines
Dermatophagoides farinae*
Ear, Middle*
Epithelial Cells*
Granulocyte-Macrophage Colony-Stimulating Factor
Humans*
Hypersensitivity
Immunity, Innate
In Vitro Techniques
Lipopolysaccharides*
Mucins
Otitis Media with Effusion
Pyroglyphidae*
Real-Time Polymerase Chain Reaction
RNA, Messenger
Toll-Like Receptors
Allergens
Cytokines
Granulocyte-Macrophage Colony-Stimulating Factor
Lipopolysaccharides
Mucins
RNA, Messenger
Toll-Like Receptors
Figure
Reference
-
1. Blair H. Natural history of childhood asthma. 20-year follow-up. Arch Dis Child. 1977; 52:613–619.2. Pedersen PA, Weeke ER. Asthma and allergic rhinitis in the same patients. Allergy. 1983; 38:25–29.3. Kim SW, Han DH, Lee SJ, Lee CH, Rhee CS. Bronchial hyperresponsiveness in pediatric rhinitis patients: the difference between allergic and nonallergic rhinitis. Am J Rhinol Allergy. 2013; 27:e63–e68.4. Döner F, Yariktas M, Demirci M. The role of allergy in recurrent otitis media with effusion. J Investig Allergol Clin Immunol. 2004; 14:154–158.5. Bernstein JM, Lee J, Conboy K, Ellis E, Li P. Further observations on the role of IgE-mediated hypersensitivity in recurrent otitis media with effusion. Otolaryngol Head Neck Surg. 1985; 93:611–615.6. Sobol SE, Taha R, Schloss MD, Mazer BD, Manoukian JJ, Tewfik TL, et al. T(H)2 cytokine expression in atopic children with otitis media with effusion. J Allergy Clin Immunol. 2002; 110:125–130.7. Wright ED, Hurst D, Miotto D, Giguere C, Hamid Q. Increased expression of major basic protein (MBP) and interleukin-5(IL-5) in middle ear biopsy specimens from atopic patients with persistent otitis media with effusion. Otolaryngol Head Neck Surg. 2000; 123:533–538.8. Hurst DS, Venge P. Evidence of eosinophil, neutrophil, and mast-cell mediators in the effusion of OME patients with and without atopy. Allergy. 2000; 55:435–441.9. Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000; 97:13766–13771.10. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001; 2:675–680.11. Kitajima T, Muroi M, Yamashita N, Tanamoto K. Toll-like receptors required for dermatophagoides farinae to activate NF-κB. Biol Pharm Bull. 2014; 37:74–80.12. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999; 274:10689–10692.13. Vlastos I, Athanasopoulos I, Mastronikolis NS, Panogeorgou T, Margaritis V, Naxakis S, et al. Impaired mucociliary clearance in allergic rhinitis patients is related to a predisposition to rhinosinusitis. Ear Nose Throat J. 2009; 88:E17–E19.14. Papadopoulos NG, Xepapadaki P, Mallia P, Brusselle G, Watelet JB, Xatzipsalti M, et al. Mechanisms of virus-induced asthma exacerbations: state-of-the-art. A GA2LEN and InterAirways document. Allergy. 2007; 62:457–470.15. Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations--a GA2 LEN-DARE systematic review. Allergy. 2011; 66:458–468.16. Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, et al. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004; 170:175–180.17. Rochlitzer S, Hoymann HG, Müller M, Braun A; U-BIOPRED consortium. No exacerbation but impaired anti-viral mechanisms in a rhinovirus-chronic allergic asthma mouse model. Clin Sci (Lond). 2014; 126:55–65.18. Rizzo MC, Naspitz CK, Fernández-Caldas E, Lockey RF, Mimiça I, Solé D. Endotoxin exposure and symptoms in asthmatic children. Pediatr Allergy Immunol. 1997; 8:121–126.19. Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996; 154:1641–1646.20. Song JJ, Lee JD, Lee BD, Chae SW, Park MK. Effect of diesel exhaust particles on human middle ear epithelial cells. Int J Pediatr Otorhinolaryngol. 2012; 76:334–338.21. Preciado D, Kuo E, Ashktorab S, Manes P, Rose M. Cigarette smoke activates NFκB-mediated Tnf-α release from mouse middle ear cells. Laryngoscope. 2010; 120:2508–2515.22. Jun HJ, Lim HW, Choi J, Jung HH, Chae SW. Ciglitazone inhibits cigarette smoke solution-induced inflammatory responses in human middle ear epithelial cells. Int J Pediatr Otorhinolaryngol. 2012; 76:1136–1139.23. Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003; 221:42–49.24. Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol. 2006; 34:661–665.25. Rose MC. Mucins: structure, function, and role in pulmonary diseases. Am J Physiol. 1992; 263:L413–L429.26. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006; 86:245–278.27. Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007; 117:1666–1676.28. Giebink GS, Le CT, Paparella MM. Epidemiology of otitis media with effusion in children. Arch Otolaryngol. 1982; 108:563–566.29. Thai P, Loukoianov A, Wachi S, Wu R. Regulation of airway mucin gene expression. Annu Rev Physiol. 2008; 70:405–429.30. Alenmyr L, Herrmann A, Högestätt ED, Greiff L, Zygmunt PM. TRPV1 and TRPA1 stimulation induces MUC5B secretion in the human nasal airway in vivo. Clin Physiol Funct Imaging. 2011; 31:435–444.31. Cho KN, Choi JY, Kim CH, Baek SJ, Chung KC, Moon UY, et al. Prostaglandin E2 induces MUC8 gene expression via a mechanism involving ERK MAPK/RSK1/cAMP response element binding protein activation in human airway epithelial cells. J Biol Chem. 2005; 280:6676–6681.32. Lee HM, Kim DH, Kim JM, Lee SH, Hwang SJ. MUC8 mucin gene up-regulation in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2004; 113:662–666.33. Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007; 87:1047–1082.34. Spannhake EW, Reddy SP, Jacoby DB, Yu XY, Saatian B, Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ Health Perspect. 2002; 110:665–670.35. Foster S, Bedford KJ, Gould ME, Coward WR, Hewitt CR. Respiratory syncytial virus infection and virus-induced inflammation are modified by contaminants of indoor air. Immunology. 2003; 108:109–115.36. Rodríguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, et al. Bacterial LPS signaling through TLR4 suppresses asthma -like response via NP synthase 2 activity. J Immunol. 2003; 171:1001–1008.37. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.38. Daan de Boer J, Roelofs JJ, de Vos AF, de Beer R, Schouten M, Hommes TJ, et al. Lipopolysaccharide inhibits Th2 lung inflammation induced by house dust mite allergens in mice. Am J Respir Cell Mol Biol. 2013; 48:382–389.39. O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999; 163:6718–6724.40. Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004; 101:4186–4191.41. Zhang J, Kumar A, Wheater M, Yu FS. Lack of MD-2 expression in human corneal epithelial cells is an underlying mechanism of lipopolysaccharide (LPS) unresponsiveness. Immunol Cell Biol. 2009; 87:141–148.42. Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999; 189:1777–1782.43. Liao EC, Hsieh CW, Chang CY, Yu SJ, Sheu ML, Wu SM, et al. Enhanced Allergic Inflammation of Der p 2 Affected by Polymorphisms of MD-2 Promoter. Allergy Asthma Immunol Res. 2015; 7:497–506.44. Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007; 316:1632–1634.45. Derewenda U, Li J, Derewenda Z, Dauter Z, Mueller GA, Rule GS, et al. The crystal structure of a major dust mite allergen Der p 2, and its biological implications. J Mol Biol. 2002; 318:189–197.46. Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007; 130:906–917.47. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.48. Yin SC, Liao EC, Chiu CL, Chang CY, Tsai JJ. Der p2 internalization by epithelium synergistically augments toll-like receptor-mediated proinflammatory signaling. Allergy Asthma Immunol Res. 2015; 7:393–403.49. Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013; 131:549–561.50. Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73:479–501.51. Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007; 447:92–96.52. Thomas WR, Smith WA, Hales BJ, Mills KL, O'Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002; 129:1–18.53. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010; 16:321–328.54. Ichikawa S, Takai T, Yashiki T, Takahashi S, Okumura K, Ogawa H, et al. Lipopolysaccharide binding of the mite allergen Der f 2. Genes Cells. 2009; 14:1055–1065.55. Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002; 347:869–877.56. Gehring U, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J. House dust endotoxin and allergic sensitization in children. Am J Respir Crit Care Med. 2002; 166:939–944.57. Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001; 358:1129–1133.58. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001; 1:69–75.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repellent effect of Mate tea and Jasmine tea against house dust mites (Dermatophagoides farinae and D. pteronyssinus)
- Evaluation of Pharmacia CAP System FEIA for House Dust Mites in Patients with Atopic Dermatitis
- Therapeutic effect of Dermatophagoides farinae antigen - autoantibody immune complex therapy in atopic dermatitis
- Secretory Differentiation of Serially Passaged Normal Human Middle Ear Epithelial(NHMEE) Cells
- The significance of specific IgE and IgG to dermatophagoides farinae according to the types of asthmatic reaction in house dust asthmatics