J Korean Acad Conserv Dent.
2009 Mar;34(2):120-129. 10.5395/JKACD.2009.34.2.120.
Histological evaluation of direct pulp capping with DSP-derived synthetic peptide in beagle dog
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Seoul National University, Korea. chobh@snu.ac.kr
- 2Department of Dental Biomaterials Science, School of Dentistry, Seoul National University, Korea.
- KMID: 2294353
- DOI: http://doi.org/10.5395/JKACD.2009.34.2.120
Abstract
- The purpose of this study was to investigate the pulpal response to direct pulp capping with dentin sialoprotein (DSP)-derived synthetic peptide in teeth of dogs, and to compare its efficacy to capping substances Ca(OH)2 and white mineral trioxide aggregate (WMTA). A total of 72 teeth of 6 healthy male beagle dogs were used. The mechanically exposed pulps were capped with one of the following: (1) DSP-derived synthetic peptide (PEP group); (2) Ca(OH)2 (CH group); (3) a mixture paste of peptide and Ca(OH)2 (PEP+CH group); or (4) white MTA (WMTA group). The access cavity was restored with a reinforced glass ionomer cement. Two dogs were sacrificed at each pre-determined intervals (2 weeks, 1 month, and 3 months). After the specimens were prepared for standard histological processing, sections were stained with hematoxylin and eosin. Under a light microscope, inflammatory response and hard tissue formation were evaluated in a blind manner by 2 observers. In the PEP group, only 3 of 17 specimens showed hard tissue formation, indication that the DSP-derived synthetic peptide did not induce proper healing of the pulp. Compared with the CH group, the PEP group demonstrated an increased inflammatory response and poor hard tissue formation. The CH and WMTA groups showed similar results for direct pulp capping in mechanically exposed teeth of dogs.
Keyword
MeSH Terms
-
Acrylic Resins
Aluminum Compounds
Animals
Calcium Compounds
Calcium Hydroxide
Dental Pulp Capping
Dentin
Dogs
Drug Combinations
Eosine Yellowish-(YS)
Extracellular Matrix Proteins
Glass Ionomer Cements
Glutamates
Guanine
Hematoxylin
Humans
Light
Male
Oligopeptides
Oxides
Phosphoproteins
Sialoglycoproteins
Silicates
Silicon Dioxide
Tooth
Pemetrexed
Acrylic Resins
Aluminum Compounds
Calcium Compounds
Calcium Hydroxide
Drug Combinations
Eosine Yellowish-(YS)
Extracellular Matrix Proteins
Glass Ionomer Cements
Glutamates
Guanine
Hematoxylin
Oligopeptides
Oxides
Phosphoproteins
Sialoglycoproteins
Silicates
Silicon Dioxide
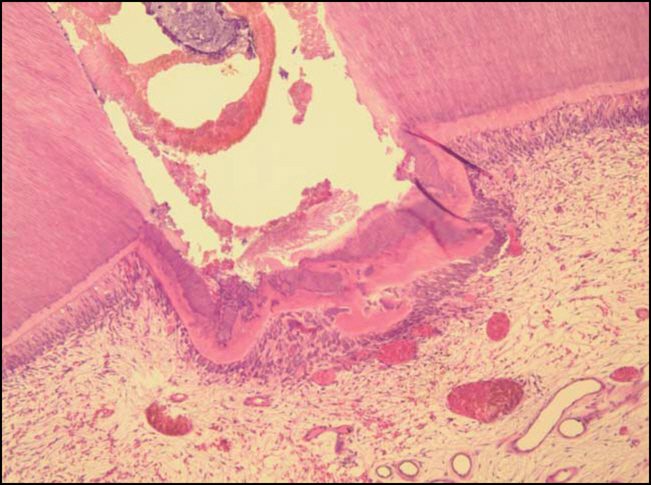
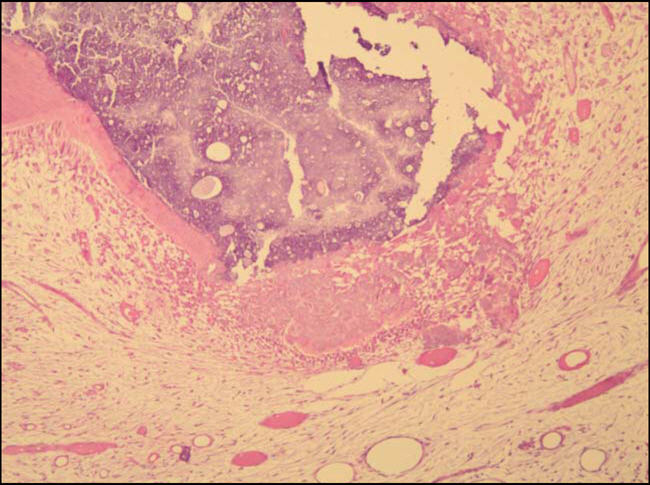
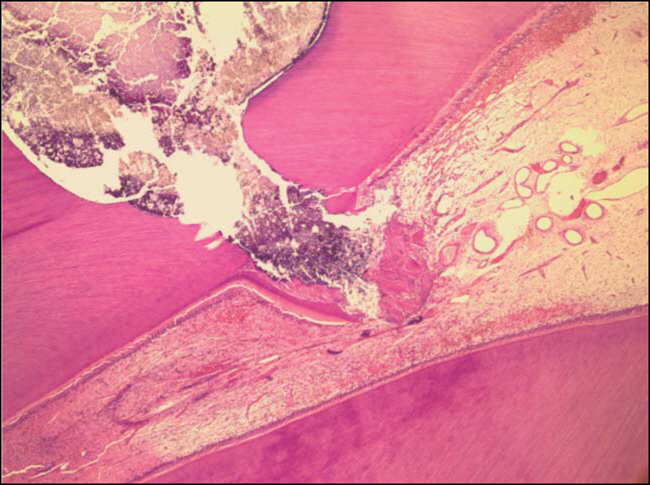
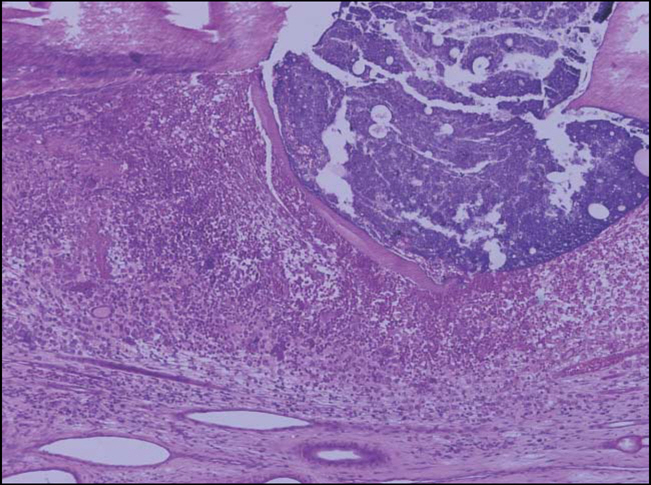
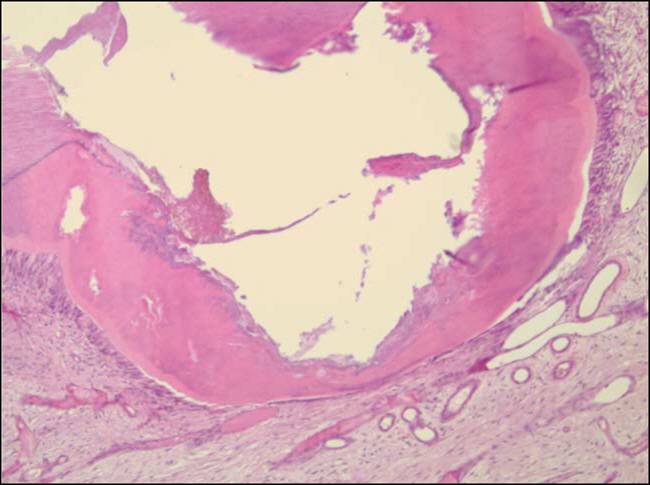
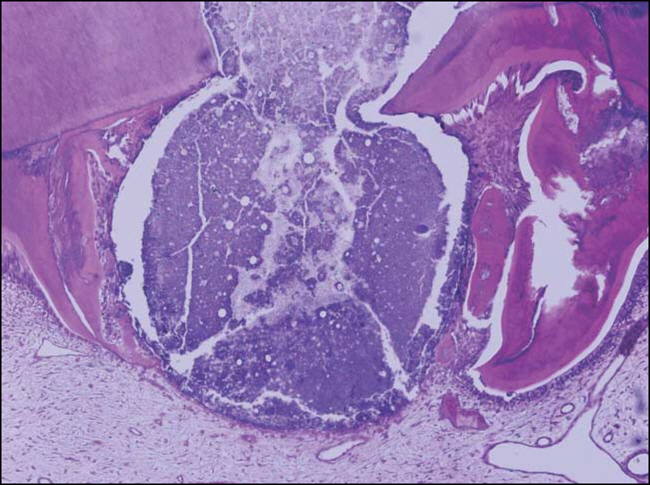
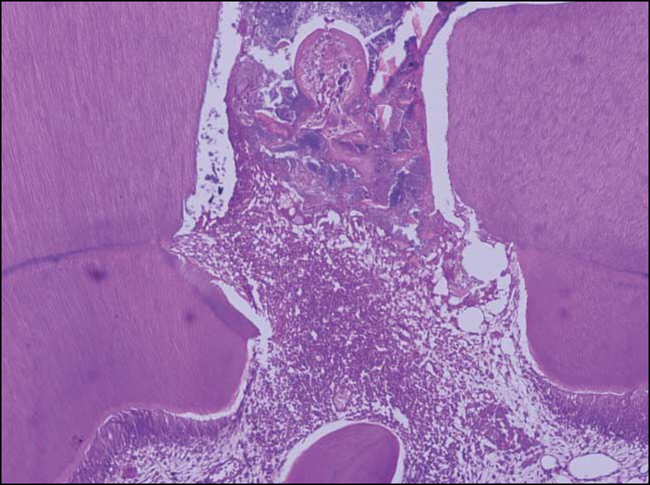
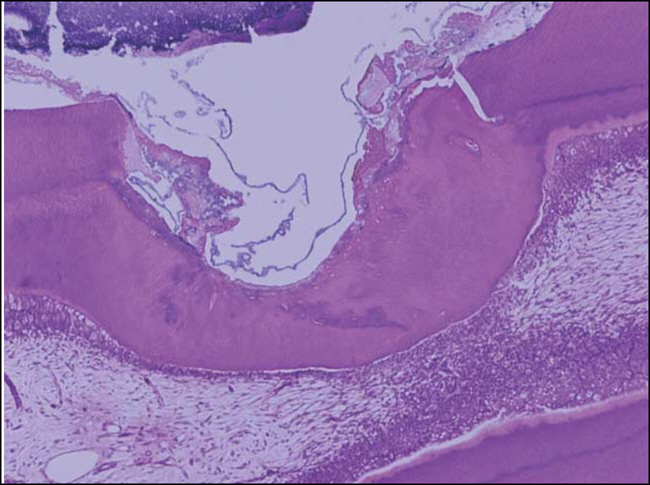
Figure
Cited by 1 articles
-
Pulp response of beagle dog to direct pulp capping materials: Histological study
Ji-Hyun Bae, Young-Gyun Kim, Pil-Young Yoon, Byeong-Hoon Cho, Yong-Hoon Choi
J Korean Acad Conserv Dent. 2010;35(1):5-12. doi: 10.5395/JKACD.2010.35.1.005.
Reference
-
References
1. Herman BW. Dentin obliteration after treatment with calcium. Zahna‥rztl Rundschau. 39:888–899. 1930; (In German).2. Schro¨der U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation and differentiation. J Dent Res. 64(suppl 1):541–548. 1985.
Article3. Pitt Ford TR, Torabinejad M, Albedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 127:1491–1494. 1996.4. Goldberg M, Six N, Decup F, Lasfargues JJ, Salih E, Tompkins K, Veis A. Bioactive molecules and the future of pulp therapy. Am J Dent. 16:66–76. 2003.5. Schuurs AHB, Gruythuysen RJM, Wesselink PR. Pulp capping with adhesive resin based composite versus calcium hydroxide: a review. Endod Dent Traumatol. 16:240–250. 2000.6. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 19:541–544. 1993.
Article7. Abedi HR, Torabinejad M, Pitt Ford TR, Bakland LK. The use of mineral trioxide aggregate cement as a direct pulp capping material. J Am Dent Assoc. 127:1491–1494. 1996.8. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 29:324–333. 2003.
Article9. Iwamoto CE, Adachi E, Pameijer CH, Barnes D, Romberg EE, Jefferies S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am J Dent. 19:85–90. 2006.10. Goldberg M, Six N, Decup F. . Application of bioactive molecules in pulp-capping situations. Adv Dent Res. 15:91–95. 2001.
Article11. Decup F, Six N, Palmier B, Buch D. Bone sialopro-tein-induced reparative dentinogenesis in the pulp of rat's molar. Clin Oral Invest. 4:110–119. 2000.
Article12. Six N, Decup F, Lasfargues JJ, Salih E, Goldberg M. Osteogenic proteins (bone sialoprotein and bone morphogenetic protein-7) and dental pulp mineralization. J Mater Sci Mater Med. 13:225–232. 2002.13. Wang J, Kennedy JG, Glimcher MJ, Salih E. Novel bioactive property of purified native bone sialoprotein in bone repair of calvarial defect. Trans Orthop Res Soc. 23:1007. 1998.14. George A, Bannon L, Sabsay B, Dillon JW, Malone J, Veis A, Jenkins NA, Gilbert DJ, Copeland NG. The carboxyl-terminal domain of phosphophoryn contains unique extended triplet amino acid repeat sequences forming ordered carboxyl-phosphate interaction ridges that may be essential in the biomineralization process. J Biol Chem. 271:32869–32873. 1996.
Article15. Qin C, Brunn JC, Ridall A. . the expression of dentin sialophosphoprotein gene in bone. J Dent Res. 81:392–394. 2002.
Article16. Butler WT. Sialoprotein of bone and dentin. J Bio Buccale. 19:83–89. 1991.17. Pitt Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trixoide aggregate as a pulp capping material. J Am Dent Assoc. 127:1491–1494. 1996.18. Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int Endod J. 36:225–231. 2002.
Article19. Nair PNR, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of health human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 41:128–150. 2008.20. Tziafas D, Kolokuris I, Alvanou A, Kaidoglou K. Short-term dentinogenic response of dog pulp tissue after its induction by demineralized or native dentine or by pre-dentine. Arch Oral Biol. 37:119–128. 1992.21. Schroder U. Evaluation of healing following experimental pulpotomy of intact human teeth and capping with calcium hydroxide. Odontol Revy. 23:329–340. 1972.22. Heithersay GS. Calcium hydroxide in the treatment of pulpless teeth with associated pathology. J Br Endod Soc. 8:74–93. 1975.
Article23. Siqueira JR, Lopez HP. Mechanism of antimicrobial activity of calcium hydroxide; a critical review. Int Endod. 32:361–369. 1999.24. Cox CF, Bergenholtz G, Heys DR, Syed SA, Fitzgerald M, Heys RJ. Pulp-capping of dental pulp mechanically exposed to oral microflora: a 1-to-2-year observation of wound healing in the monkey. J Oral Pathol. 14:156–168. 1985.25. Stanley HR, Pameijer CH. Dentistry's friend, calcium hydroxide. Oper Dent. 22:1–3. 1997.26. Cox CF, Bergenholtz G, Fitzgerald M, Heys DR, Heys RJ, Avery JK, Baker JA. Capping of the dental pulp mechanically exposed to the oral microflora: 5-week observation of wound healing in the monkey. J Oral Pathol. 11:327–339. 1982.27. Holland R, De souza V, Nevy NJ, Otobone Filho JA, Bernabe DF, Dezan Jr E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydoxide. J Endod. 25:161–166. 1999.28. Torabinejad M, Watson EF, Pitt Ford TR. Sealing ability of mineral trioxide aggregate when used as a root end filling material. J Endod. 19:591–595. 1993.29. Fischer EJ, Arens DE, Miller CH. Bacterial leakage of mineral trioxide aggregate as compared with zinc-free amalgam, intermediate restorative material, and Super-EBA as a root-end filling material. J Endod. 24:176–179. 1998.
Article30. Wu MK, Kontakiotis EG, Wesselink PR. Long-term seal provided by some root-end fillng materials. J Endod. 24:557–560. 1998.31. Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histological assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 23:225–228. 1997.32. Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabe PF, Dezan Jr E. Reaction of dog's teeth to root canal filling with mineral trioxide aggregate or a glass ionomer sealer. J Endod. 25:728–730. 1999.
Article33. Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fiboroblasts. J Endod. 26:288–291. 2000.34. Myers K, Kaminski E, Lautenschalger E, Miller D. The effects of mineral trioxide aggregate on the dog pulp (Abstract). J Endod. 22:198. 1996.35. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 102:809–815. 2006.
Article36. Agamy HA, Bakry NS, Mounir MF, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp-capping agents in pulpotomized primary teeth. Pediatr Dent. 26:302–309. 2004.37. Maroto M, Barberia E, Vera V, Garcia-Godoy F. Dentin bridge formation after white trioxide aggregate (white MTA) pulpotomies in primary molars. Am J Dent. 19:75–79. 2006.38. Parirokh M, Asgary S, Eghbal MJ, Stowe S, Eslami B, Eskandarizade A, Shabahang S. A comparative study of white and grey mineral trioxide aggregate as pulp capping agents in dog's teeth. Dent Traumatol. 21:150–154. 2005.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulp response of beagle dog to direct pulp capping materials: Histological study
- Histological Study of Reparative Dentin Formation after Direct Pulp Capping and Pulpotomy using MTA
- Effect of Mineral Trioxide Aggregate and Calcium Hydroxide on Reparative Dentin Formation in Rats
- Considerations during crown reattachment procedure over the pulpal exposure: case report
- Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo