Restor Dent Endod.
2021 May;46(2):e17. 10.5395/rde.2021.46.e17.
Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Dental Science Research Institute, Chonnam National University, Gwangju, Korea
- 2Department of Pharmacology and Dental Therapeutics, School of Dentistry, Dental Science Research Institute, Chonnam National University, Gwangju, Korea
- KMID: 2548061
- DOI: http://doi.org/10.5395/rde.2021.46.e17
Abstract
Objectives
In recent in vitro study, it was reported that osteostatin (OST) has an odontogenic effect and synergistic effect with mineral trioxide aggregate (MTA) in human dental pulp cells. Therefore, the aim of this study was to evaluate whether OST has a synergistic effect with MTA on hard tissue formation in vivo.
Materials and Methods
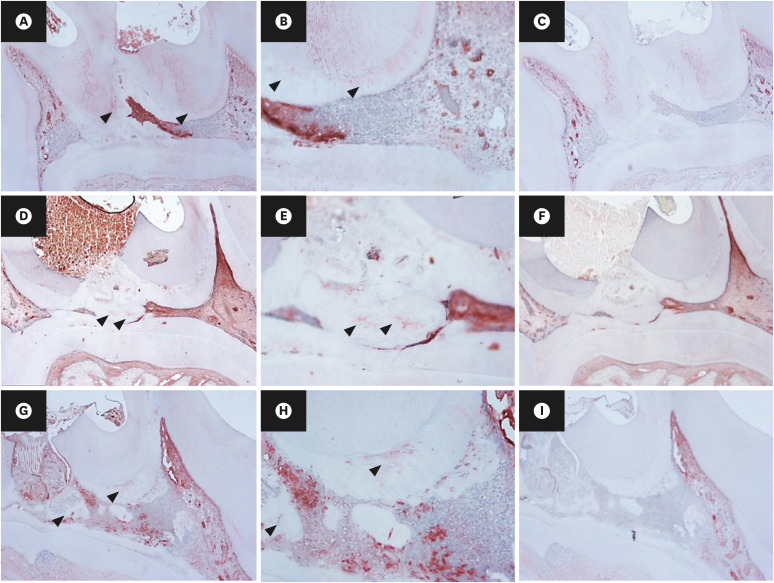
Thirty-two maxillary molars of Spraque-Dawley rats were used in this study. An occlusal cavity was prepared and the exposed pulps were randomly divided into 3 groups: group 1 (control; ProRoot MTA), group 2 (OST 100 μM + ProRoot MTA), group 3 (OST 10 mM + ProRoot MTA). Exposed pulps were capped with each material and cavities were restored with resin modified glass ionomer. The animals were sacrificed after 4 weeks. All harvested teeth were scanned with micro-computed tomography (CT). The samples were prepared and hard tissue formation was evaluated histologically. For immunohistochemical analysis, the specimens were sectioned and incubated with primary antibodies against dentin sialoprotein (DSP).
Results
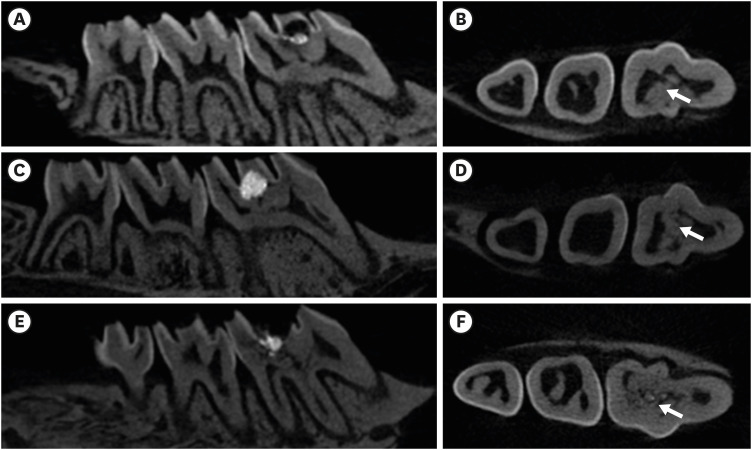
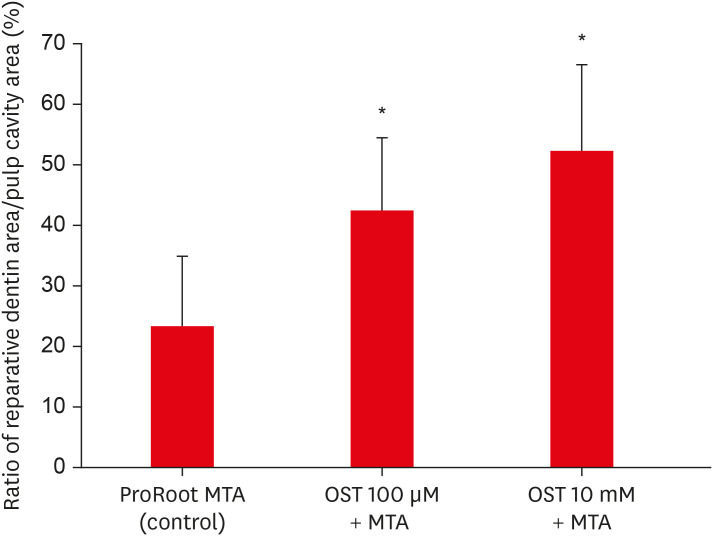
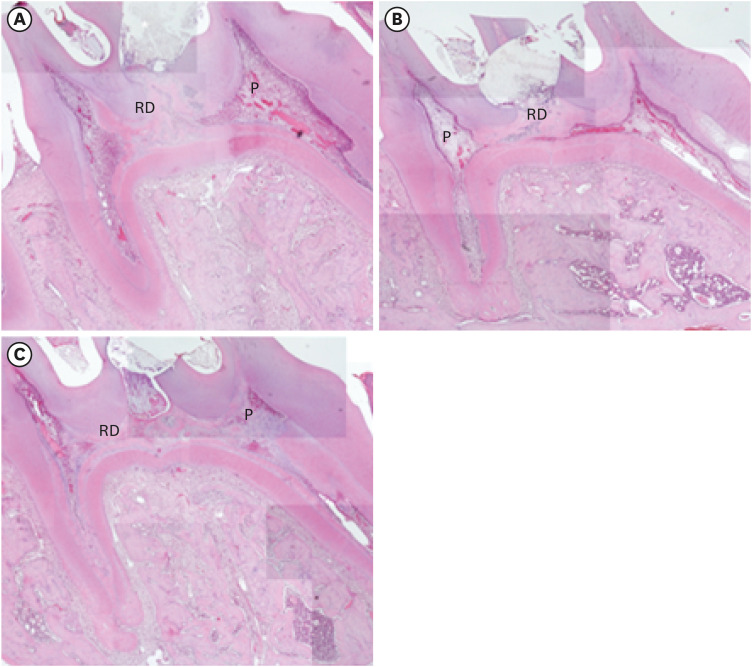
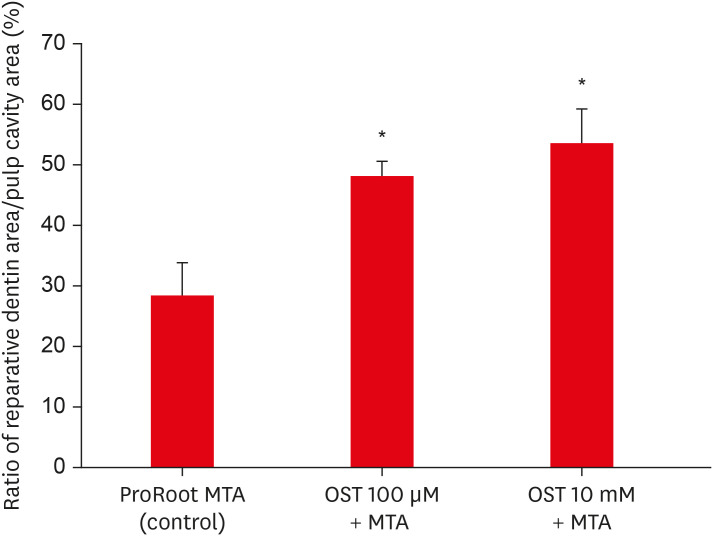
In the micro-CT analysis, it is revealed that OST with ProRoot MTA groups showed more mineralized bridge than the control (p < 0.05). In the H&E staining, it is showed that more quantity of the mineralized dentin bridge was formed in the OST with ProRoot MTA group compared to the control (p < 0.05). In all groups, DSP was expressed in newly formed reparative dentin area.
Conclusions
OST can be a supplementary pulp capping material when used with MTA to make synergistic effect in hard tissue formation.
Figure
Reference
-
1. An S, Gao Y, Ling J, Wei X, Xiao Y. Calcium ions promote osteogenic differentiation and mineralization of human dental pulp cells: implications for pulp capping materials. J Mater Sci Mater Med. 2012; 23:789–795. PMID: 22190198.
Article2. Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 2009; 34:615–625. PMID: 19830978.
Article3. Scarano A, Manzon L, Di Giorgio R, Orsini G, Tripodi D, Piattelli A. Direct capping with four different materials in humans: histological analysis of odontoblast activity. J Endod. 2003; 29:729–734. PMID: 14651279.
Article4. Hörsted-Bindslev P, Vilkinis V, Sidlauskas A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 96:591–600. PMID: 14600695.
Article5. Casella G, Ferlito S. The use of mineral trioxide aggregate in endodontics. Minerva Stomatol. 2006; 55:123–143. PMID: 16575384.6. Fuks AB. Vital pulp therapy with new materials for primary teeth: new directions and treatment perspectives. Pediatr Dent. 2008; 30:211–219. PMID: 18615986.
Article7. Modena KC, Casas-Apayco LC, Atta MT, Costa CA, Hebling J, Sipert CR, Navarro MF, Santos CF. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci. 2009; 17:544–554. PMID: 20027424.
Article8. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413. PMID: 20171353.
Article9. Gelman R, Park H. Pulp revascularization in an immature necrotic tooth: a case report. Pediatr Dent. 2012; 34:496–499. PMID: 23265169.10. Kang JY, Lee BN, Son HJ, Koh JT, Kang SS, Son HH, Chang HS, Hwang IN, Hwang YC, Oh WM. Biocompatibility of mineral trioxide aggregate mixed with hydration accelerators. J Endod. 2013; 39:497–500. PMID: 23522544.
Article11. Yun HM, Chang SW, Park KR, Herr L, Kim EC. Combined effects of growth hormone and mineral trioxide aggregate on growth, differentiation, and angiogenesis in human dental pulp cells. J Endod. 2016; 42:269–275. PMID: 26435469.
Article12. Min KS, Yang SH, Kim EC. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endod. 2009; 35:847–851. PMID: 19482184.
Article13. Liu CH, Huang TH, Hung CJ, Lai WY, Kao CT, Shie MY. The synergistic effects of fibroblast growth factor-2 and mineral trioxide aggregate on an osteogenic accelerator in vitro. Int Endod J. 2014; 47:843–853. PMID: 24319992.
Article14. Woo SM, Kim WJ, Lim HS, Choi NK, Kim SH, Kim SM, Jung JY. Combination of mineral trioxide aggregate and platelet-rich fibrin promotes the odontoblastic differentiation and mineralization of human dental pulp cells via BMP/Smad signaling pathway. J Endod. 2016; 42:82–88. PMID: 26364004.
Article15. Martin TJ. Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol Rev. 2016; 96:831–871. PMID: 27142453.
Article16. Lozano D, Sánchez-Salcedo S, Portal-Núñez S, Vila M, López-Herradón A, Ardura JA, Mulero F, Gómez-Barrena E, Vallet-Regí M, Esbrit P. Parathyroid hormone-related protein (107-111) improves the bone regeneration potential of gelatin-glutaraldehyde biopolymer-coated hydroxyapatite. Acta Biomater. 2014; 10:3307–3316. PMID: 24704694.
Article17. Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996; 76:127–173. PMID: 8592727.
Article18. Cornish J, Callon KE, Nicholson GC, Reid IR. Parathyroid hormone-related protein-(107-139) inhibits bone resorption in vivo . Endocrinology. 1997; 138:1299–1304. PMID: 9048639.
Article19. Cornish J, Callon KE, Lin C, Xiao C, Moseley JM, Reid IR. Stimulation of osteoblast proliferation by C-terminal fragments of parathyroid hormone-related protein. J Bone Miner Res. 1999; 14:915–922. PMID: 10352099.
Article20. Lozano D, Manzano M, Doadrio JC, Salinas AJ, Vallet-Regí M, Gómez-Barrena E, Esbrit P. Osteostatin-loaded bioceramics stimulate osteoblastic growth and differentiation. Acta Biomater. 2010; 6:797–803. PMID: 19716446.
Article21. Lozano D, Feito MJ, Portal-Núñez S, Lozano RM, Matesanz MC, Serrano MC, Vallet-Regí M, Portolés MT, Esbrit P. Osteostatin improves the osteogenic activity of fibroblast growth factor-2 immobilized in Si-doped hydroxyapatite in osteoblastic cells. Acta Biomater. 2012; 8:2770–2777. PMID: 22487933.
Article22. Han JW, Lee BN, Kim SM, Koh JT, Min KS, Hwang YC. Odontogenic potential of parathyroid hormone–related protein (107-111) alone or in combination with mineral trioxide aggregate in human dental pulp cells. J Endod. 2017; 43:2054–2060. PMID: 29061354.
Article23. Kim J, Song YS, Min KS, Kim SH, Koh JT, Lee BN, Chang HS, Hwang IN, Oh WM, Hwang YC. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor Dent Endod. 2016; 41:29–36. PMID: 26877988.
Article24. Holland R, de Souza V, de Mello W, Nery MJ, Bernabé PF, Otoboni Filho JA. Permeability of the hard tissue bridge formed after pulpotomy with calcium hydroxide: a histologic study. J Am Dent Assoc. 1979; 99:472–475. PMID: 112134.
Article25. Nyborg H. Healing processes in the pulp on capping; a morphologic study; experiments on surgical lesions of the pulp in dog and man. Acta Odontol Scand. 1955; 13(Supplement 16):1–130.26. Masterton JB. The healing of wounds of the dental pulp. An investigation of the nature of the scar tissue and of the phenomena leading to its formation. Dent Pract Dent Rec. 1966; 16:325–339. PMID: 4956604.27. Kozlov M, Massler M. Histologic effects of various drugs on amputated pulps of rat molars. Oral Surg Oral Med Oral Pathol. 1960; 13:455–469. PMID: 14411548.
Article28. Butler WT. Dentin matrix proteins and dentinogenesis. Connect Tissue Res. 1995; 33:59–65. PMID: 7554963.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histological Study of Reparative Dentin Formation after Direct Pulp Capping and Pulpotomy using MTA
- Pulp response of beagle dog to direct pulp capping materials: Histological study
- Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry
- Effect of Mineral Trioxide Aggregate and Calcium Hydroxide on Reparative Dentin Formation in Rats
- Pulp response of mineral trioxide aggregate, calcium sulfate or calcium hydroxide