Transl Clin Pharmacol.
2016 Jun;24(2):84-89. 10.12793/tcp.2016.24.2.84.
Pharmacokinetic comparison of two levofloxacin 100-mg tablet formulations and determination of time point appropriately reflecting its area under the curve
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Kyung Hee University College of Medicine and Hospital, Seoul 02447, Republic of Korea. ysvin@khu.ac.kr
- KMID: 2290474
- DOI: http://doi.org/10.12793/tcp.2016.24.2.84
Abstract
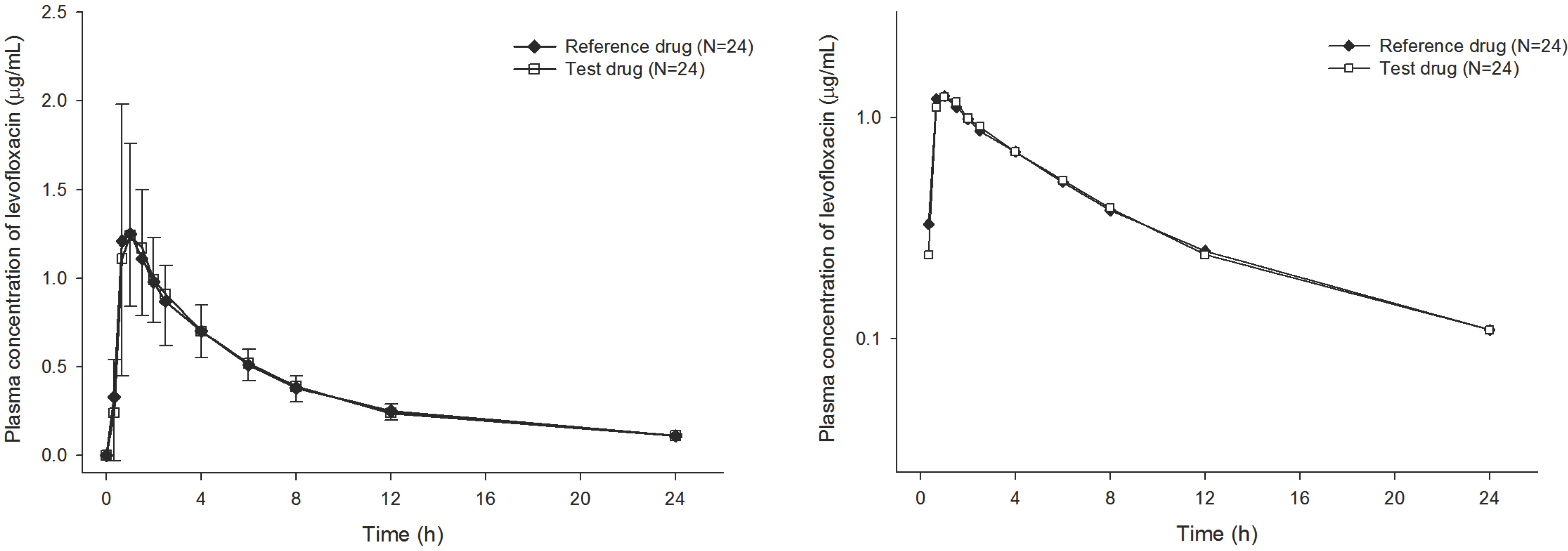
- Levofloxacin is a broad-spectrum antibiotic with activity against gram-positive and -negative bacteria. This study compared the pharmacokinetics (PK) and evaluated the bioequivalence of two levofloxacin 100-mg tablet formulations. An open, randomized, two-way crossover study was conducted in 28 healthy volunteers. The reference (Cravit Tab 100-mg, Jeil) or test (Levobacter Tab, Seoul) formulation was administered and serial blood samples were collected over 24 h for PK analysis. Levofloxacin plasma concentrations were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The correlation of levofloxacin concentration at various time points with the area under the concentration time-curve over the time interval from 0 extrapolated to infinity (AUCinf) was estimated to determine the best reflected time point. The average half-life, maximum plasma concentration (Cmax), and AUClast were comparable. The 90% confidence intervals (CIs) of the geometric mean ratio (GMR test/reference) of AUClast and Cmax were 0.8200-1.0633 and 0.9474-1.0643 respectively. Both formulations were tolerated with no clinically relevant safety issues. Plasma levofloxacin concentrations at various time points correlated well with the AUCinf, and showed high correlation coefficients (r > 0.7, P < 0.001) for both drugs 8 and 12 h after administration. Both formulations showed similar PK profiles while levofloxacin plasma levels after administration indicated their bioequivalence. The Cmax and AUClast GMR 90% CIs were 0.80-1.25. Moreover, 12 h was the best time point to predict the AUCinf and therefore suitable for therapeutic drug monitoring.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Galan-Herrera JF, Poo JL, Rosales-Sanchez O, Fuentes-Fuentes E, Cariño L, Burke-Fraga V, et al. Bioavailability of two oral formulations of a single dose of levofloxacin 500 mg: An open-label, randomized, two-period crossover comparison in healthy Mexican volunteers. Clin Ther. 2009; 31:1796–1803. doi: 10.1016/j.clinthera.2009.08.004.
Article2. Shi SJ, Han ZM, Chen HT, Zeng FD. Pharmacokinetics and bioequivalence of levofloxacin in healthy Chinese subject. Asian Journal of Pharmacodynamics and Pharmacokinetics. 2009; 9:313–318.3. Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997; 32:101–119.
Article4. Tsaganos T, Kouki P, Digenis P, Giamarellou H, Giamarellos-Bourboulis EJ, Kanellakopoulou K. Pharmacokinetics of levofloxacin after single and multiple oral doses in patients undergoing intermittent haemodialysis. Int J Antimicrob Agents. 2008; 32:46–49. doi: 10.1016/j.ijantimicag.2008.02.011.
Article5. Domján A, Kakuk P, Sándor J. The Helsinki Declaration at 50 years: comments on the 2013 modifications. Lege Artis Med. 2014; 24:152–158.6. Chow SC, Wang H. On sample size calculation in bioequivalence trials. J Pharmacokinet Pharmacodyn. 2001; 28:155–169.7. Niioka T, Uno T, Yasui-Furukori N, Shimizu M, Sugawara K, Tateishi T. Identification of the time-point which gives a plasma rabeprazole concentration that adequately reflects the area under the concentration-time curve. Eur J Clin Pharmacol. 2006; 62:855–861.
Article8. Guidance for Industry. Bioavailability and Bioequivalence studies for orally administered drug products –General considerations. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf/AccessedOct15. 2015.9. Slavik RS, Jewesson PJ. Selecting antibacterials for outpatient parenteral antimicrobial therapy. Clin Pharmacokinet. 2003; 42:793–817.
Article10. Minimum Requirements for Bioequivalence Test. http://www.mfds.go.kr/index.do?mid=1013&seq=8562&cmd=v. Accessed Oct 15. 2015.11. Wimer SM, Schoonover L, Garrison MW. Levofloxacin: A therapeutic review. Clin Ther. 1998; 20:1049–1070.
Article12. Wispelwey B. Clinical implications of pharmacokinetics and pharmacodynamics of fluoroquinolones. Clin Infect Dis. 2005; 41:S127–S135.
Article13. Millan X, Muggia V, Ostrowsky B. Antimicrobial agents, drug adverse reactions and interactions, and cancer. Cancer Treat Res. 2014; 161:413–462. doi: 10.1007/978-3-319-04220-6_14.
Article14. Macgowan AP, Wootton M, Holt HA. The antibacterial efficacy of levofloxacin and ciprofloxacin against Pseudomonas aeruginosa assessed by combining antibiotic exposure and bacterial susceptibility. J Antimicrob Chemother. 1999; 43:345–349.15. Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and metaanalysis. Lancet Infect Dis. 2009; 9:153–161. doi: 10.1016/S1473-3099 (09)70041-6.
Article16. WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. http://apps.who.int/iris/bitstream/10665/44597/1/9789241501583_eng.pdf. Accessed Feb 26. 2016.17. Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, et al. Population pharmacokinetics of levofloxacin, gatifloxacin and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2008; 52:852–857.
Article18. Stambaugh JJ, Berning SE, Bulpitt AE, Hollender ES, Narita M, Ashkin D, et al. Ofloxacin population pharmacokinetics in patients with tuberculosis. Int J Tuberc Lung Dis. 2002; 6:503–509.
Article19. Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002; 62:2169–2183.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A bioequivalence study of two levofloxacin tablets in healthy male subjects
- Bioequivalence and Dose Proportionality of Olmesartan Medoxomil Formulations
- Safety and pharmacokinetic comparison between fenofibric acid 135 mg capsule and 110 mg entericcoated tablet in healthy volunteers
- Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers
- Pharmacokinetic comparison of two valproic acid formulations: a plain and a controlled release enteric-coated tablets