J Bone Metab.
2014 Aug;21(3):163-167. 10.11005/jbm.2014.21.3.163.
Factors and Mechanisms Involved in the Coupling from Bone Resorption to Formation: How Osteoclasts Talk to Osteoblasts
- Affiliations
-
- 1Department of Bone and Joint Disease, National Center for Geriatrics and Gerontology, Obu, Japan. kikeda@ncgg.go.jp
- KMID: 2286286
- DOI: http://doi.org/10.11005/jbm.2014.21.3.163
Abstract
- Bone remodeling is the fundamental means by which the quality as well as quantity of the skeleton is maintained throughout adult life. When bone remodeling goes awry, a metabolic bone disease such as osteoporosis ensues. Among multiple phases of the complex remodeling process, we focus in this review on factors and mechanisms that are involved in the coupling of bone formation to preceding resorption.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Characteristics of contact and distance osteogenesis around modified implant surfaces in rabbit tibiae
Jung-Yoo Choi, Jae-Hyuk Sim, In-Sung Luke Yeo
J Periodontal Implant Sci. 2017;47(3):182-191. doi: 10.5051/jpis.2017.47.3.182.
Reference
-
1. Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007; 13:791–801.
Article2. Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994; 55:273–286.
Article3. Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009; 15:757–765.
Article4. Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012; 18:1095–1101.
Article5. Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005; 11:76–81.
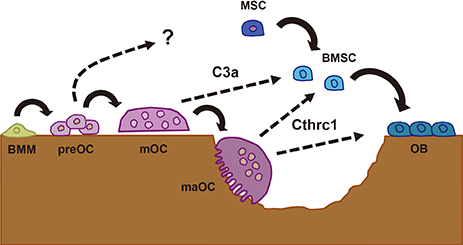
Article6. Matsuoka K, Park KA, Ito M, et al. Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J Bone Miner Res. 2014; 29:1522–1530.
Article7. Takeshita S, Fumoto T, Matsuoka K, et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest. 2013; 123:3914–3924.
Article8. Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006; 4:111–121.
Article9. Ryu J, Kim HJ, Chang EJ, et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006; 25:5840–5851.
Article10. Pederson L, Ruan M, Westendorf JJ, et al. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008; 105:20764–20769.
Article11. Lotinun S, Kiviranta R, Matsubara T, et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013; 123:666–681.
Article12. Kubota K, Sakikawa C, Katsumata M, et al. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Miner Res. 2002; 17:257–265.
Article13. Sanchez-Fernandez MA, Gallois A, Riedl T, et al. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One. 2008; 3:e3537.
Article14. Pyagay P, Heroult M, Wang Q, et al. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005; 96:261–268.
Article15. Kimura H, Kwan KM, Zhang Z, et al. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS One. 2008; 3:e3174.
Article16. Yamamoto S, Nishimura O, Misaki K, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008; 15:23–36.
Article17. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013; 19:179–192.
Article18. Ehrnthaller C, Ignatius A, Gebhard F, et al. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011; 17:317–329.
Article19. Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011; 17:1674–1679.
Article20. Negishi-Koga T, Shinohara M, Komatsu N, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011; 17:1473–1480.
Article21. Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res. 2001; 16:1583–1585.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biological characteristics of osteoporosis drugs: the effect of osteoblast–osteoclast coupling
- Bone Cell Communication Factors Provide a New Therapeutic Strategy for Osteoporosis
- An electron microscopic study on the alveolar bone remodelling in pressure zones of rat molar periodontium associated with orthodontic tooth movement
- Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption
- Pathobiology of Paget's Disease of Bone