Korean J Physiol Pharmacol.
2014 Oct;18(5):403-409. 10.4196/kjpp.2014.18.5.403.
Bis is Induced by Oxidative Stress via Activation of HSF1
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. leejh@catholic.ac.kr
- 2Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 3Cancer Evolution Research Center, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2285539
- DOI: http://doi.org/10.4196/kjpp.2014.18.5.403
Abstract
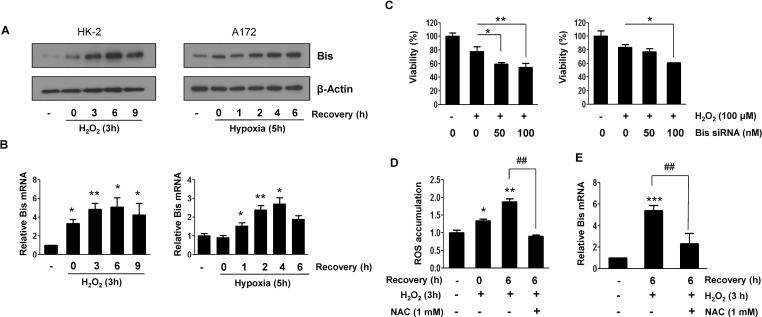
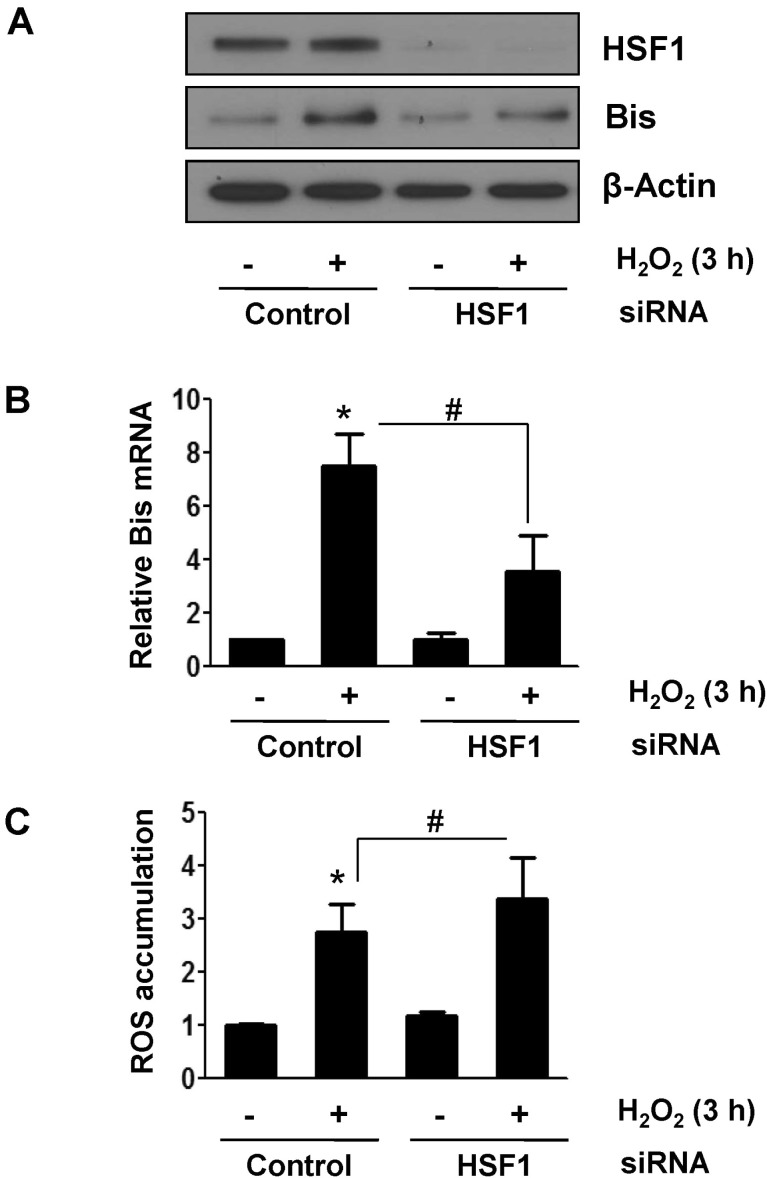
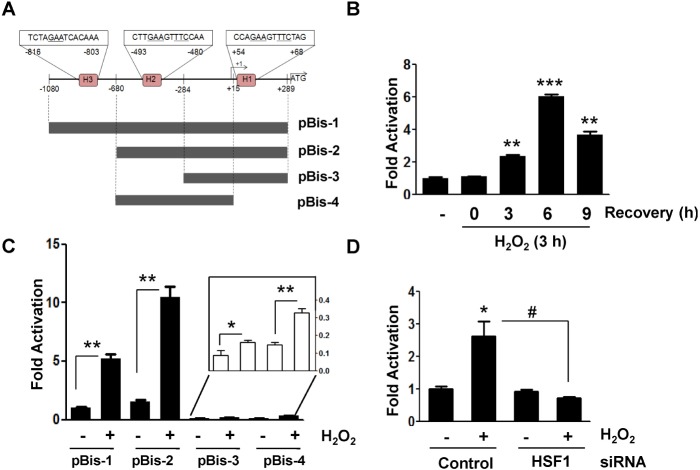
- The Bis protein is known to be involved in a variety of cellular processes including apoptosis, migration, autophagy as well as protein quality control. Bis expression is induced in response to a number of types of stress, such as heat shock or a proteasome inhibitor via the activation of heat shock factor (HSF)1. We report herein that Bis expression is increased at the transcriptional level in HK-2 kidney tubular cells and A172 glioma cells by exposure to oxidative stress such as H2O2 treatment and oxygen-glucose deprivation, respectively. The pretreatment of HK-2 cells with N-acetyl cysteine, suppressed Bis induction. Furthermore, HSF1 silencing attenuated Bis expression that was induced by H2O2, accompaniedby increase in reactive oxygen species (ROS) accumulation. Using a series of deletion constructs of the bis gene promoter, two putative heat shock elements located in the proximal region of the bis gene promoter were found to be essential for the constitutive expression is as well as the inducible expression of Bis. Taken together, our results indicate that oxidative stress induces Bis expression at the transcriptional levels via activation of HSF1, which might confer an expansion of antioxidant capacity against pro-oxidant milieu. However, the possible role of the other cis-element in the induction of Bis remains to be determined.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999; 18:6183–6190. PMID: 10597216.
Article2. Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999; 274:781–786. PMID: 9873016.
Article3. Doong H, Price J, Kim YS, Gasbarre C, Probst J, Liotta LA, Blanchette J, Rizzo K, Kohn E. CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-gamma and Hsp70/Hsc70. Oncogene. 2000; 19:4385–4395. PMID: 10980614.4. Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complexmediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003; 278:28490–28500. PMID: 12750378.5. Kassis JN, Guancial EA, Doong H, Virador V, Kohn EC. CAIR-1/BAG-3 modulates cell adhesion and migration by downregulating activity of focal adhesion proteins. Exp Cell Res. 2006; 312:2962–2971. PMID: 16859681.
Article6. Suzuki M, Iwasaki M, Sugio A, Hishiya A, Tanaka R, Endo T, Takayama S, Saito T. BAG3 (BCL2-associated athanogene 3) interacts with MMP-2 to positively regulate invasion by ovarian carcinoma cells. Cancer Lett. 2011; 303:65–71. PMID: 21316839.
Article7. Fontanella B, Birolo L, Infusini G, Cirulli C, Marzullo L, Pucci P, Turco MC, Tosco A. The co-chaperone BAG3 interacts with the cytosolic chaperonin CCT: new hints for actin folding. Int J Biochem Cell Biol. 2010; 42:641–650. PMID: 20018251.
Article8. Iwasaki M, Tanaka R, Hishiya A, Homma S, Reed JC, Takayama S. BAG3 directly associates with guanine nucleotide exchange factor of Rap1, PDZGEF2, and regulates cell adhesion. Biochem Biophys Res Commun. 2010; 400:413–418. PMID: 20800573.
Article9. Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008; 283:1437–1444. PMID: 18006506.
Article10. Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009; 28:889–901. PMID: 19229298.
Article11. Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, Galbiati M, Fontana E, Marino M, Carra S, Bendotti C, De Biasi S, Poletti A. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet. 2010; 19:3440–3456. PMID: 20570967.
Article12. Seo YJ, Jeon MH, Lee JH, Lee YJ, Youn HJ, Ko JH, Lee JH. Bis induces growth inhibition and differentiation of HL-60 cells via up-regulation of p27. Exp Mol Med. 2005; 37:624–630. PMID: 16391524.
Article13. Yoon JS, Lee MY, Lee JS, Park CS, Youn HJ, Lee JH. Bis is involved in glial differentiation of p19 cells induced by retinoic Acid. Korean J Physiol Pharmacol. 2009; 13:251–256. PMID: 19885044.
Article14. De Marco M, Turco MC, Rosati A. BAG3 protein is induced during cardiomyoblast differentiation and modulates myogenin expression. Cell Cycle. 2011; 10:850–852. PMID: 21311226.
Article15. Zhu H, Liu P, Li J. BAG3: a new therapeutic target of human cancers? Histol Histopathol. 2012; 27:257–261. PMID: 22237703.16. Pagliuca MG, Lerose R, Cigliano S, Leone A. Regulation by heavy metals and temperature of the human BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 2003; 541:11–15. PMID: 12706811.
Article17. Chen L, Wu W, Dentchev T, Zeng Y, Wang J, Tsui I, Tobias JW, Bennett J, Baldwin D, Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004; 79:239–247. PMID: 15325571.
Article18. Tabuchi Y, Ando H, Takasaki I, Feril LB Jr, Zhao QL, Ogawa R, Kudo N, Tachibana K, Kondo T. Identification of genes responsive to low intensity pulsed ultrasound in a human leukemia cell line Molt-4. Cancer Lett. 2007; 246:149–156. PMID: 16678341.
Article19. Wang HQ, Liu HM, Zhang HY, Guan Y, Du ZX. Transcriptional upregulation of BAG3 upon proteasome inhibition. Biochem Biophys Res Commun. 2008; 365:381–385. PMID: 17996194.
Article20. Jacobs AT, Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem. 2009; 284:9176–9183. PMID: 19179333.
Article21. Song S, Kole S, Precht P, Pazin MJ, Bernier M. Activation of heat shock factor 1 plays a role in pyrrolidine dithiocarbamatemediated expression of the co-chaperone BAG3. Int J Biochem Cell Biol. 2010; 42:1856–1863. PMID: 20692357.
Article22. Franceschelli S, Rosati A, Lerose R, De Nicola S, Turco MC, Pascale M. Bag3 gene expression is regulated by heat shock factor 1. J Cell Physiol. 2008; 215:575–577. PMID: 18286539.
Article23. Du ZX, Zhang HY, Meng X, Gao YY, Zou RL, Liu BQ, Guan Y, Wang HQ. Proteasome inhibitor MG132 induces BAG3 expression through activation of heat shock factor 1. J Cell Physiol. 2009; 218:631–637. PMID: 19006120.
Article24. Gentilella A, Passiatore G, Deshmane S, Turco MC, Khalili K. Activation of BAG3 by Egr-1 in response to FGF-2 in neuroblastoma cells. Oncogene. 2008; 27:5011–5018. PMID: 18469860.
Article25. Li C, Li S, Kong DH, Meng X, Zong ZH, Liu BQ, Guan Y, Du ZX, Wang HQ. BAG3 is upregulated by c-Jun and stabilizes JunD. Biochim Biophys Acta. 2013; 1833:3346–3354. PMID: 24140207.
Article26. Gentilella A, Khalili K. Autoregulation of co-chaperone BAG3 gene transcription. J Cell Biochem. 2009; 108:1117–1124. PMID: 19777443.
Article27. Lee MY, Kim SY, Shin SL, Choi YS, Lee JH, Tsujimoto Y, Lee JH. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol. 2002; 175:338–346. PMID: 12061864.
Article28. Cho KO, Lee KE, Youn DY, Jeong KH, Kim JY, Yoon HH, Lee JH, Kim SY. Decreased vulnerability of hippocampal neurons after neonatal hypoxia-ischemia in bis-deficient mice. Glia. 2012; 60:1915–1929. PMID: 22907804.29. Jung SE, Kim YK, Youn DY, Lim MH, Ko JH, Ahn YS, Lee JH. Down-modulation of Bis sensitizes cell death in C6 glioma cells induced by oxygen-glucose deprivation. Brain Res. 2010; 1349:1–10. PMID: 20599823.
Article30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.31. Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995; 11:441–469. PMID: 8689565.
Article32. Lim JH, Youn DY, Yoo HJ, Yoon HH, Kim MY, Chung S, Kim YS, Chang YS, Park CW, Lee JH. Aggravation of diabetic nephropathy in BCL-2 interacting cell death suppressor (BIS)-haploinsufficient mice together with impaired induction of superoxide dismutase (SOD) activity. Diabetologia. 2014; 57:214–223. PMID: 24078136.
Article33. Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011; 80:1089–1115. PMID: 21417720.
Article34. Gidalevitz T, Prahlad V, Morimoto RI. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb Perspect Biol. 2011; 3:pii: a009704.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERK-mediated phosphorylation of BIS regulates nuclear translocation of HSF1 under oxidative stress
- Effect of BIS depletion on HSF1-dependent transcriptional activation in A549 non-small cell lung cancer cells
- Epilepsy and Oxidative Stress
- Roles of the complement system in alcohol-induced liver disease
- Oxidative stress and endometriosis