Korean J Physiol Pharmacol.
2014 Oct;18(5):383-390. 10.4196/kjpp.2014.18.5.383.
The Effects of Eupatilin (Stillen(R)) on Motility of Human Lower Gastrointestinal Tracts
- Affiliations
-
- 1Division of Colorectal Surgery, Department of Surgery, Seoul National University College of Medicine, Seoul 110-744, Korea. kjparkmd@plaza.snu.ac.kr
- 2Department of Physiology, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 3Healthcare Research Institute, Seoul National University Hospital Healthcare System Gangnam Center, Seoul 135-984, Korea.
- 4Dong-A, Pharmaceutical Co, Seoul 130-823, Korea.
- KMID: 2285536
- DOI: http://doi.org/10.4196/kjpp.2014.18.5.383
Abstract
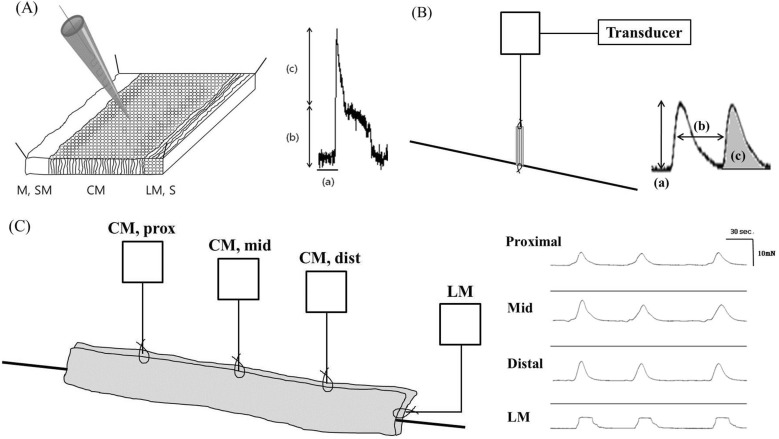
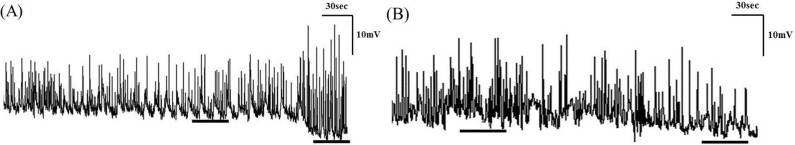
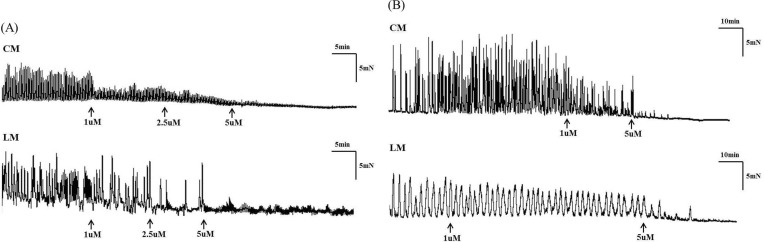
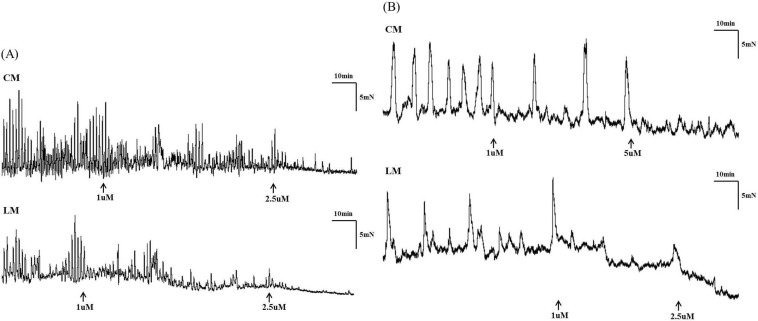
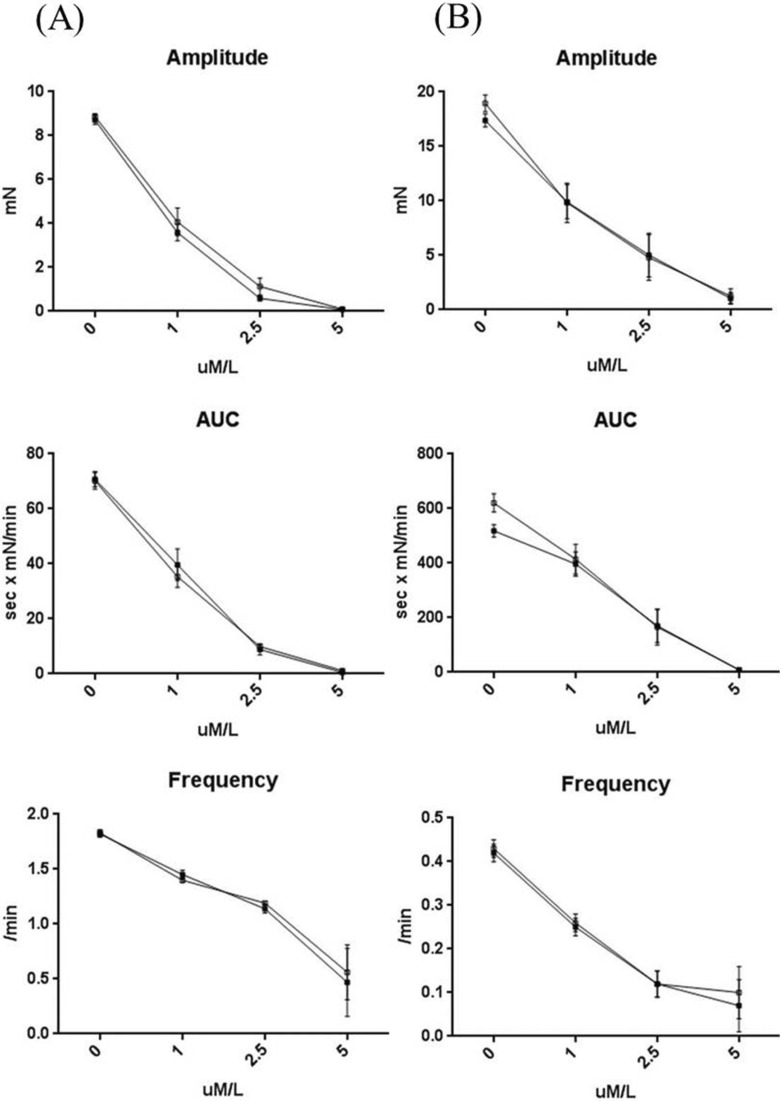
- Gastrointestinal motility consists of phasic slow-wave contractions and the migrating motor complex (MMC). Eupatilin (Stillen(R)) has been widely used to treat gastritis and peptic ulcers, and various cytokines and neuropeptides are thought to be involved, which can affect gastrointestinal motility. We performed a study to identify the effects of eupatilin on lower gastrointestinal motility with electromechanical recordings of smooth muscles in the human ileum and colon. Ileum and colon samples were obtained from patients undergoing bowel resection. The tissues were immediately stored in oxygenated Krebs-Ringer's bicarbonate solution, and conventional microelectrode recordings from muscle cells and tension recordings from muscle strips and ileal or colonic segments were performed. Eupatilin was perfused into the tissue chamber, and changes in membrane potentials and contractions were measured. Hyperpolarization of resting membrane potential (RMP) was observed after administration of eupatilin. The amplitude, AUC, and frequency of tension recordings from circular and longitudinal smooth muscle strips and bowel segments of the ileum and colon were significantly decreased after admission of eupatilin. Eupatilin elicited dose-dependent decreases during segmental tension recordings. In conclusion, eupatilin (Stillen(R)) showed inhibitory effects on the human ileum and colon. We propose that this drug may be useful for treating diseases that increase bowel motility, but further studies are necessary.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The hepato-protective effect of eupatilin on an alcoholic liver disease model of rats
Hak Yeong Lee, Yoonjin Nam, Won Seok Choi, Tae Wook Kim, Jaehwi Lee, Uy Dong Sohn
Korean J Physiol Pharmacol. 2020;24(5):385-394. doi: 10.4196/kjpp.2020.24.5.385.Electrophysiological and Mechanical Characteristics in Human Ileal Motility: Recordings of Slow Waves Conductions and Contractions,
In vitro
Seung-Bum Ryoo, Heung-Kwon Oh, Sang Hui Moon, Eun Kyung Choe, Sung A Yu, Sung-Hye Park, Kyu Joo Park
Korean J Physiol Pharmacol. 2015;19(6):533-542. doi: 10.4196/kjpp.2015.19.6.533.
Reference
-
1. Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996; 111:492–515. PMID: 8690216.
Article2. Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997; 9:99–107. PMID: 9198085.
Article3. Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006; 290:C1411–C1427. PMID: 16381798.
Article4. Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007; 132:1852–1865. PMID: 17484879.
Article5. Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007; 133:907–917. PMID: 17678922.
Article6. Choe EK, Moon JS, Moon SB, So IS, Park KJ. Electromechanical characteristics of the human colon in vitro: is there any difference between the right and left colon? Int J Colorectal Dis. 2010; 25:1117–1126. PMID: 20544209.
Article7. Kim BJ, Park KJ, Kim HW, Choi S, Jun JY, Chang IY, Jeon JH, So I, Kim SJ. Identification of TRPM7 channels in human intestinal interstitial cells of Cajal. World J Gastroenterol. 2009; 15:5799–5804. PMID: 19998500.8. Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. 1997; 18:393–403. PMID: 9357324.
Article9. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491. PMID: 16678561.
Article10. Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987; 92:1885–1893. PMID: 3569764.
Article11. Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Effects of alosetron on spontaneous migrating motor complexes in murine small and large bowel in vitro. Am J Physiol Gastrointest Liver Physiol. 2001; 281:G974–G983. PMID: 11557518.
Article12. Seol SY, Kim MH, Rew JS, Choi MG. A phase III clinical trial of stillen(TM) for erosive gastritis. Korean J Gastrointest Endosc. 2004; 28:230–236.13. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001; 49:364–371. PMID: 11511558.
Article14. Lee EB, Cheon SA, Lee ES, Kim KK, Ko ST, Yu KJ, Shin DS, Kang SY, Kim SH, Sohn MH. General pharmacology of artemisia extract powder, DA-9601. J Appl Pharmacol. 1996; 4:174–183.15. Oh TY, Ahn BO, Ko JI, Ryu BK, Son MW, Kim SH, Kim WB, Lee EB. Studies on Protective effect of da-9601, an artemisiae herba extract, against ethanol-induced gastric mucosal damage and its mechanism. J Appl Pharmacol. 1997; 5:202–210.16. Oh TY, Ryu BK, Ko JI, Ahn BO, Kim SH, Kim WB, Lee EB, Jin JH, Hahm KB. Protective effect of DA-9601, an extract ofArtemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 1997; 20:414–419. PMID: 18982482.
Article17. Ahn BO, Ryu BK, Ko JI, Oh TY, Kim SH, Kim WB, Yang J, Lee eB, Hahm KB. Beneficial effect of DA-9601, an extract of artemisiae herba, on animals models of inflammatory bowel disease. J Appl Pharmacol. 1997; 5:165–173.18. Lee JJ, Han BG, Kim MN, Chung MH. The inhibitory effect of eupatilin on Helicobacter pylori-induced release of leukotriene D4 in the human neutrophils and gastric mucosal cells. Korean J Physiol Pharmacol. 1997; 1:573–580.19. Seo HJ, Park KK, Han SS, Chung WY, Son MW, Kim WB, Surh YJ. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. Int J Cancer. 2002; 100:456–462. PMID: 12115530.20. Kim DH, Na HK, Oh TY, Kim WB, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem Pharmacol. 2004; 68:1081–1087. PMID: 15313404.
Article21. Kim MJ, Kim DH, Na HK, Oh TY, Shin CY, Surh Ph D Professor YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS) cells. J Environ Pathol Toxicol Oncol. 2005; 24:261–269. PMID: 16393120.
Article22. Fukunaga Y, Mine Y, Yoshikawa S, Takeuchi T, Hata F, Yagasaki O. Role of prostacyclin in acetylcholine release from myenteric plexus of guinea-pig ileum. Eur J Pharmacol. 1993; 233:237–242. PMID: 8467869.
Article23. Xiao ZL, Biancani P, Behar J. Effects of progesterone on motility and prostaglandin levels in the distal guinea pig colon. Am J Physiol Gastrointest Liver Physiol. 2009; 297:G886–G893. PMID: 20501437.
Article24. Manning BP, Sharkey KA, Mawe GM. Effects of PGE2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G1388–G1397. PMID: 12388206.
Article25. Konturek SK, Konturek PC. Role of nitric oxide in the digestive system. Digestion. 1995; 56:1–13. PMID: 7895925.
Article26. Boeckxstaens GE, Pelckmans PA, Bult H, De Man JG, Herman AG, Van Maercke YM. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990; 190:239–246. PMID: 1981752.
Article27. DuPont AW, Jiang ZD, Harold SA, Snyder N, Galler GW, Garcia-Torres F, DuPont HL. Motility abnormalities in irritable bowel syndrome. Digestion. 2014; 89:119–123. PMID: 24503633.
Article28. American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009; 104(Suppl 1):S1–35. PMID: 19521341.29. Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001; 96:1499–1506. PMID: 11374689.
Article30. Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation-and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006; 130:34–43. PMID: 16401466.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastrointestinal Motility Modulating Drugs
- A Phase III Clinical Trial of Stillen(TM) for Erosive Gastritis
- The Advantages and Disadvantages of Prokinetics
- Reduced risk of gastrointestinal bleeding associated with eupatilin in aspirin plus acid suppressant users: nationwide population-based study
- Eupatilin Inhibits Gastric Cancer Cell Growth by Blocking STAT3-Mediated VEGF Expression