Korean J Physiol Pharmacol.
2014 Aug;18(4):341-346. 10.4196/kjpp.2014.18.4.341.
Effects of Lubiprostone on Pacemaker Activity of Interstitial Cells of Cajal from the Mouse Colon
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chosun University, Gwangju 501-759, Korea. jyjun@chosun.ac.kr
- 2Department of Radiology, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- KMID: 2285529
- DOI: http://doi.org/10.4196/kjpp.2014.18.4.341
Abstract
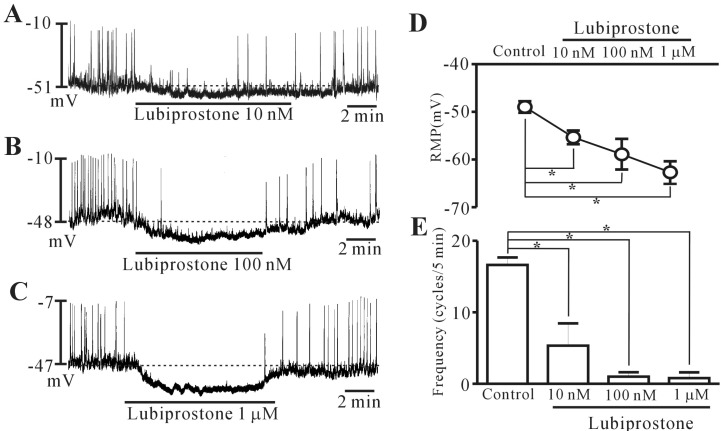
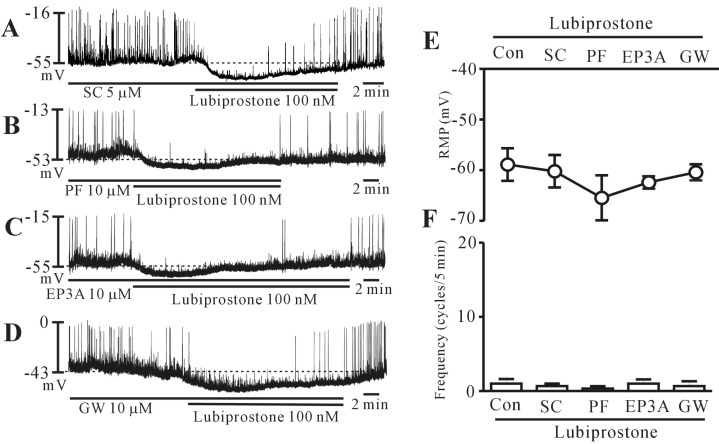
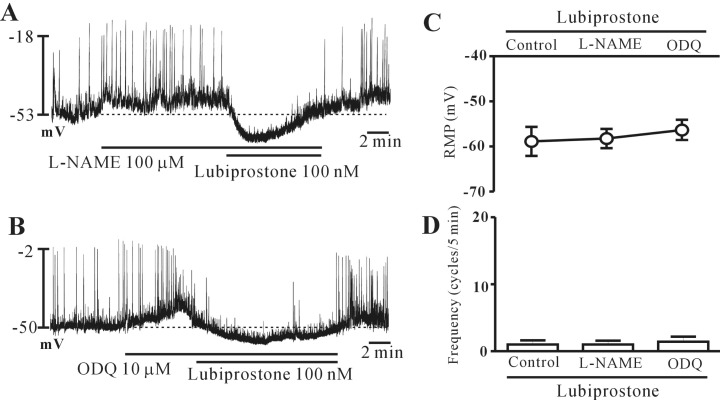
- Lubiprostone is a chloride (Cl-) channel activator derived from prostaglandin E1 and used for managing constipation. In addition, lubiprostone affects the activity of gastrointestinal smooth muscles. Interstitial cells of Cajal (ICCs) are pacemaker cells that generate slow-wave activity in smooth muscles. We studied the effects of lubiprostone on the pacemaker potentials of colonic ICCs. We used the whole-cell patch-clamp technique to determine the pacemaker activity in cultured colonic ICCs obtained from mice. Lubiprostone hyperpolarized the membrane and inhibited the generation of pacemaker potentials. Prostanoid EP1, EP2, EP3, and EP4 antagonists (SC-19220, PF-04418948, 6-methoxypyridine-2-boronc acid N-phenyldiethanolamine ester, and GW627368, respectively) did not block the response to lubiprostone. L-NG-nitroarginine methyl ester (L-NAME, an inhibitor of nitric oxide synthase) and 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ, an inhibitor of guanylate cyclase) did not block the response to lubiprostone. In addition, tetraethylammonium (TEA, a voltage-dependent potassium [K+] channel blocker) and apamin (a calcium [Ca2+]-dependent K+ channel blocker) did not block the response to lubiprostone. However, glibenclamide (an ATP-sensitive K+ channel blocker) blocked the response to lubiprostone. Similar to lubiprostone, pinacidil (an opener of ATP-sensitive K+ channel) hyperpolarized the membrane and inhibited the generation of pacemaker potentials, and these effects were inhibited by glibenclamide. These results suggest that lubiprostone can modulate the pacemaker potentials of colonic ICCs via activation of ATP-sensitive K+ channel through a prostanoid EP receptor-independent mechanism.
MeSH Terms
Figure
Reference
-
1. McKeage K, Plosker GL, Siddiqui MA. Lubiprostone. Drugs. 2006; 66:873–879. PMID: 16706562.
Article2. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009; 10:143–152. PMID: 19236188.
Article3. Carter NJ, Scott LJ. Lubiprostone: in constipation-predominant irritable bowel syndrome. Drugs. 2009; 69:1229–1237. PMID: 19537839.4. Schey R, Rao SS. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig Dis Sci. 2011; 56:1619–1625. PMID: 21523369.
Article5. Crowell MD, Harris LA, DiBaise JK, Olden KW. Activation of type-2 chloride channels: a novel therapeutic target for the treatment of chronic constipation. Curr Opin Investig Drugs. 2007; 8:66–70.6. Bijvelds MJ, Bot AG, Escher JC, De Jonge HR. Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009; 137:976–985. PMID: 19454284.7. Fei G, Wang YZ, Liu S, Hu HZ, Wang GD, Qu MH, Wang XY, Xia Y, Sun X, Bohn LM, Cooke HJ, Wood JD. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G823–G832. PMID: 19179625.
Article8. Norimatsu Y, Moran AR, MacDonald KD. Lubiprostone activates CFTR, but not ClC-2, via the prostaglandin receptor (EP(4)). Biochem Biophys Res Commun. 2012; 426:374–379. PMID: 22960173.
Article9. Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, Shao JZ, Lavins BJ, Currie MG, Fitch DA, Jeglinski BI, Eng P, Fox SM, Johnston JM. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011; 365:527–536. PMID: 21830967.
Article10. Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci. 2011; 56:339–351. PMID: 21140215.11. Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007; 41:345–351. PMID: 17413599.12. Cuppoletti J, Malinowska DH, Chakrabarti J, Ueno R. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat. 2008; 86:56–60. PMID: 18440264.
Article13. Camilleri M, Bharucha AE, Ueno R, Burton D, Thomforde GM, Baxter K, McKinzie S, Zinsmeister AR. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G942–G947. PMID: 16603730.
Article14. Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008; 154:126–135. PMID: 18332851.
Article15. Chan WW, Mashimo H. Lubiprostone Increases Small Intestinal Smooth Muscle Contractions Through a Prostaglandin E Receptor 1 (EP1)-mediated Pathway. J Neurogastroenterol Motil. 2013; 19:312–318. PMID: 23875097.16. Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998; 4:848–851. PMID: 9662380.
Article17. Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006; 68:307–343. PMID: 16460275.
Article18. Kim BJ, Kwon YK, Kim E, So I. Effects of histamine on cultured interstitial cells of cajal in murine small intestine. Korean J Physiol Pharmacol. 2013; 17:149–156. PMID: 23626477.
Article19. Rumessen JJ, Thuneberg L. Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scand J Gastroenterol Suppl. 1996; 216:82–94. PMID: 8726282.
Article20. Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006; 576:653–658. PMID: 16916909.
Article21. Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003; 98:618–624. PMID: 12650797.
Article22. Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008; 20(Suppl 1):54–63. PMID: 18402642.
Article23. Dey I, Lejeune M, Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol. 2006; 149:611–623. PMID: 17016496.24. Mizumori M, Akiba Y, Kaunitz JD. Lubiprostone stimulates duodenal bicarbonate secretion in rats. Dig Dis Sci. 2009; 54:2063–2069. PMID: 19657734.
Article25. Cuthbert AW. Lubiprostone targets prostanoid EP4 receptors in ovine airways. Br J Pharmacol. 2011; 162:508–520. PMID: 20883477.26. Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006; 18:187–198. PMID: 17167224.27. Shahi PK, Choi S, Jeong YJ, Park CG, So I, Jun JY. Basal cGMP regulates the resting pacemaker potential frequency of cultured mouse colonic interstitial cells of Cajal. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387:641–648. PMID: 24676911.
Article28. Fujita A, Takeuchi T, Saitoh N, Hanai J, Hata F. Expression of Ca2+-activated K+ channels, SK3, in the interstitial cells of Cajal in the gastrointestinal tract. Am J Physiol Cell Physiol. 2001; 281:C1727–C1733. PMID: 11600437.29. Lyford GL, Farrugia G. Ion channels in gastrointestinal smooth muscle and interstitial cells of Cajal. Curr Opin Pharmacol. 2003; 3:583–587. PMID: 14644008.
Article30. Nakayama S, Ohya S, Liu HN, Watanabe T, Furuzono S, Wang J, Nishizawa Y, Aoyama M, Murase N, Matsubara T, Ito Y, Imaizumi Y, Kajioka S. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J Cell Sci. 2005; 118:4163–4173. PMID: 16141235.31. Park CG, Kim YD, Kim MY, Kim JS, Choi S, Yeum CH, Parajuli SP, Park JS, Jeong HS, So I, Kim KW, Jun JY. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007; 376:175–184. PMID: 17932655.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Capsaicin Inhibits the Spontaneous Pacemaker Activity in Interstitial Cells of Cajal From the Small Intestine of Mouse
- Overexpression of Stromal Interaction Molecule 1/Store-operated Calcium Entry-associated Regulatory Factor in Interstitial Cells of Cajal in Mouse Jejunum Impairs Pacemaker Activity
- Effects of ATP on Pacemaker Activity of Interstitial Cells of Cajal from the Mouse Small Intestine
- Interplay of Hydrogen Sulfide and Nitric Oxide on the Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Pituitary Adenylate Cyclase-activating Polypeptide Inhibits Pacemaker Activity of Colonic Interstitial Cells of Cajal