Chonnam Med J.
2011 Aug;47(2):72-79. 10.4068/cmj.2011.47.2.72.
Interplay of Hydrogen Sulfide and Nitric Oxide on the Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chosun University, Gwangju, Korea. jyjun@chosun.ac.kr
- KMID: 2048788
- DOI: http://doi.org/10.4068/cmj.2011.47.2.72
Abstract
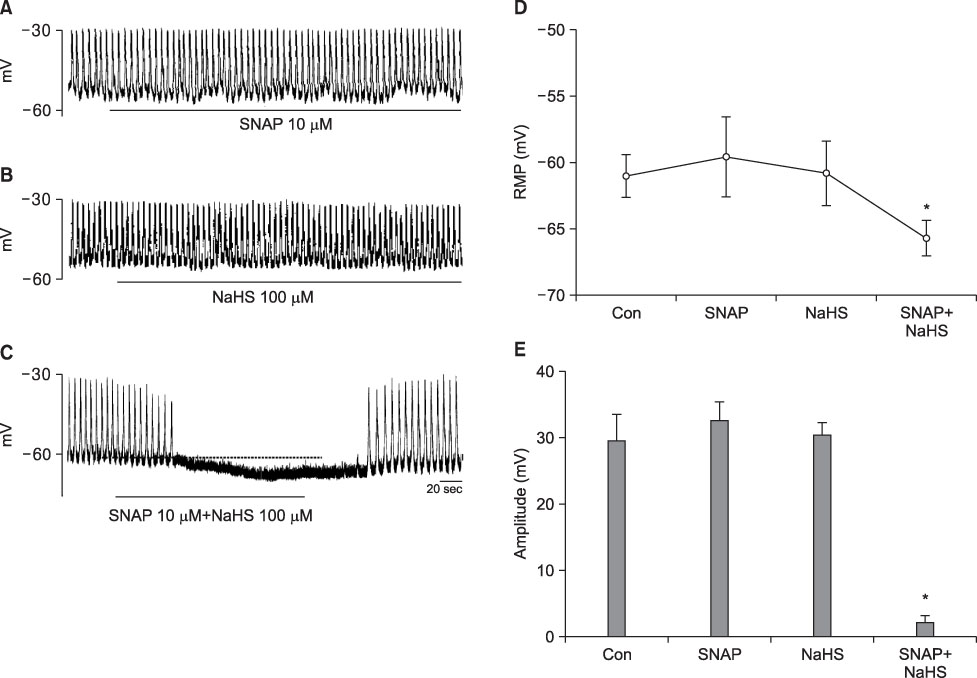
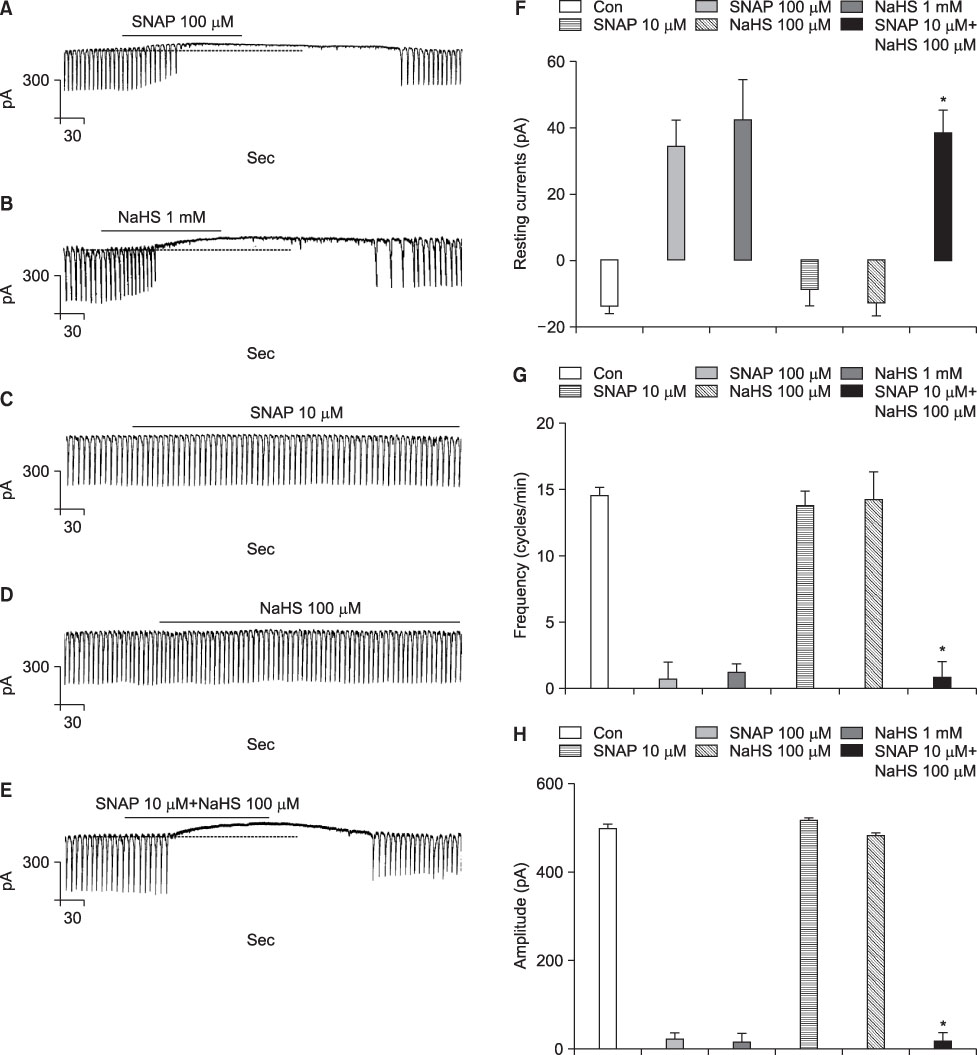
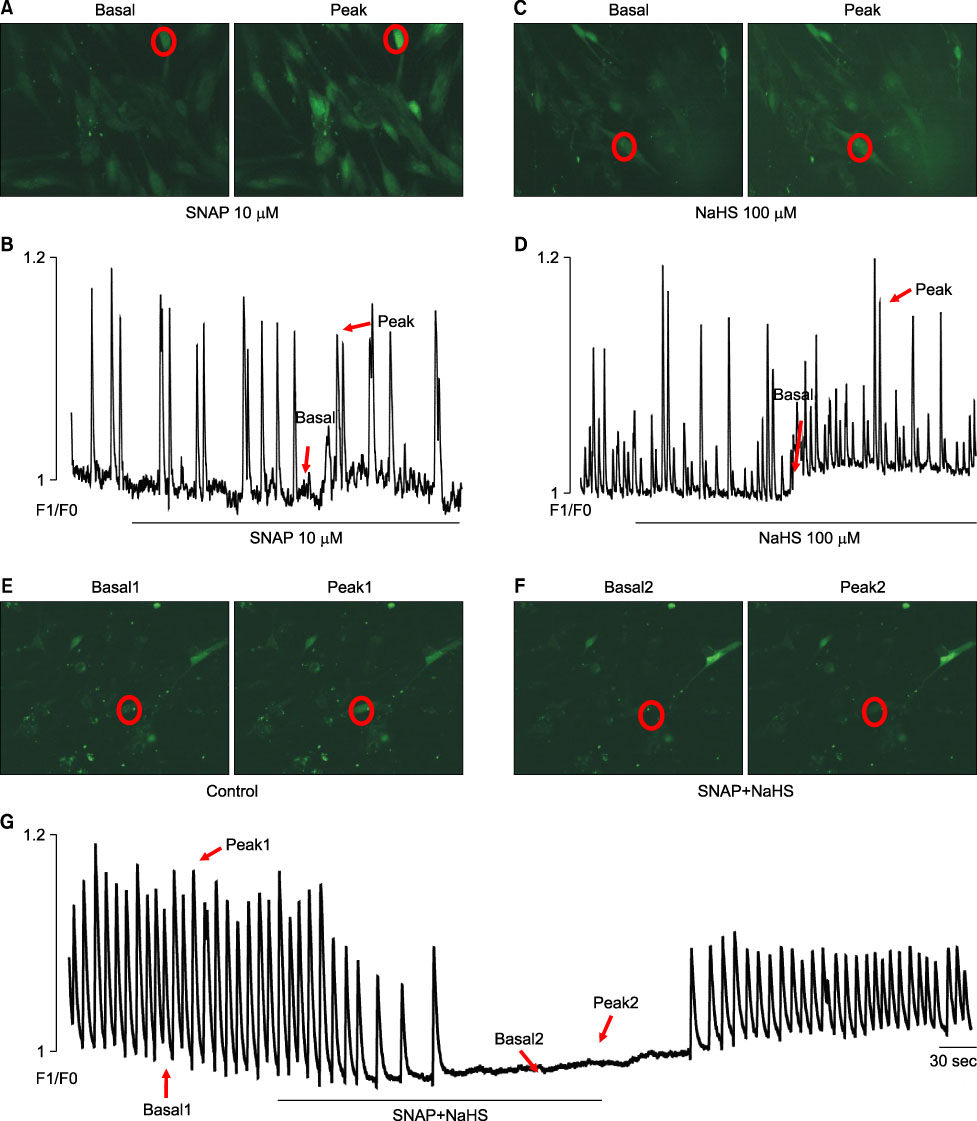
- We studied whether nitric oxide (NO) and hydrogen sulfide (H2S) have an interaction on the pacemaker activities of interstitial cells of Cajal (ICC) from the mouse small intestine. The actions of NO and H2S on pacemaker activities were investigated by using the whole-cell patch-clamp technique and intracellular Ca2+ analysis at 30degrees C in cultured mouse ICC. Exogenously applied (+/-)-S-nitroso-N-acetylpenicillamine (SNAP), an NO donor, or sodium hydrogen sulfide (NaHS), a donor of H2S, showed no influence on pacemaker activity (potentials and currents) in ICC at low concentrations (10 microM SNAP and 100 microM NaHS), but SNAP or NaHS completely inhibited pacemaker amplitude and pacemaker frequency with increases in the resting currents in the outward direction at high concentrations (SNAP 100 microM and NaHS 1 mM). Co-treatment with 10 microM SNAP plus 100 microM NaHS also inhibited pacemaker amplitude and pacemaker frequency with increases in the resting currents in the outward direction. ODQ, a guanylate cyclase inhibitor, or glibenclamide, an ATP-sensitive K+ channel inhibitor, blocked the SNAP+NaHS-induced inhibition of pacemaker currents in ICC. Also, we found that SNAP+NaHS inhibited the spontaneous intracellular Ca2+ ([Ca2+]i) oscillations in cultured ICC. In conclusion, this study describes the enhanced inhibitory effects of NO plus H2S on ICC in the mouse small intestine. NO+H2S inhibited the pacemaker activity of ICC by modulating intracellular Ca2+. These results may be evidence of a physiological interaction of NO and H2S in ICC for modulating gastrointestinal motility.
Keyword
MeSH Terms
Figure
Reference
-
1. Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein RJ, et al. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989. 13:105–109.
Article2. Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989. 38:973–981.3. Savage JC, Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr. 1990. 526:540–545.
Article4. Sanders KM, Ward SM. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992. 262:G379–G392.
Article5. Ueno T, Duenes JA, Zarroug AE, Sarr MG. Nitrergic mechanisms mediating inhibitory control of longitudinal smooth muscle contraction in mouse small intestine. J Gastrointest Surg. 2004. 8:831–841.
Article6. Bukovska G, Kery V, Kraus JP. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Expr Purif. 1994. 5:442–448.
Article7. Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990. 269:335–340.
Article8. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997. 237:527–531.
Article9. Gallego D, Clavé P, Donovan J, Rahmati R, Grundy D, Jiménez M, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008. 20:1306–1316.
Article10. Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995. 269:C1577–C1585.
Article11. Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008. 295:H1330–H1340.
Article12. Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, et al. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation. 2009. 120:888–896.
Article13. Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006. 40:119–130.
Article14. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001. 20:6008–6016.
Article15. Parajuli SP, Choi S, Lee J, Kim YD, Park CG, Kim MY, et al. The inhibitory effects of hydrogen sulfide on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2010. 14:83–89.
Article16. Park CG, Kim YD, Kim MY, Kim JS, Choi S, Yeum CH, et al. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007. 376:175–184.
Article17. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997. 237:527–531.
Article18. Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation--a tale of three gases! Pharmacol Ther. 2009. 123:386–400.
Article19. Jun JY, Choi S, Chang IY, Yoon CK, Jeong HG, Kong ID, et al. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005. 144:242–251.
Article20. Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, et al. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006. 18:187–198.
Article21. Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993. 7:328–338.
Article22. Sergeant GP, Johnston L, McHale NG, Thornbury KD, Hollywood MA. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol. 2006. 574:167–181.
Article23. Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, et al. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000. 525:105–111.
Article24. Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000. 525:355–361.
Article25. Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002. 29:312–316.
Article26. Small RC, Berry JL, Foster RW. Potassium channel opening drugs and the airways. Braz J Med Biol Res. 1992. 25:983–998.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Capsaicin Inhibits the Spontaneous Pacemaker Activity in Interstitial Cells of Cajal From the Small Intestine of Mouse
- The Inhibitory Effects of Hydrogen Sulfide on Pacemaker Activity of Interstitial Cells of Cajal from Mouse Small Intestine
- Inhibition of Pacemaker Activity of Interstitial Cells of Cajal by Hydrogen Peroxide via Activating ATP-sensitive K(+) Channels
- Slow Wave Activity and Modulations in Mouse Jejunum Myenteric Plexus In Situ
- Effects of ATP on Pacemaker Activity of Interstitial Cells of Cajal from the Mouse Small Intestine