Korean J Physiol Pharmacol.
2015 Sep;19(5):435-440. 10.4196/kjpp.2015.19.5.435.
Pituitary Adenylate Cyclase-activating Polypeptide Inhibits Pacemaker Activity of Colonic Interstitial Cells of Cajal
- Affiliations
-
- 1Department of Physiology, Chonnam National University Medical School, Gwangju 501-757, Korea. parkjs@jnu.ac.kr
- 2Department of Pathology, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 3Department of Internal Medicine, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 4Department of Dermatology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong 445-907, Korea.
- 5Department of Physiology, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- KMID: 2285588
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.435
Abstract
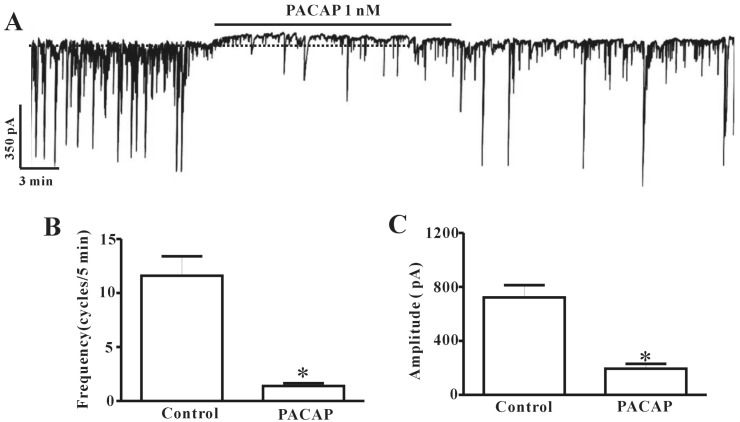
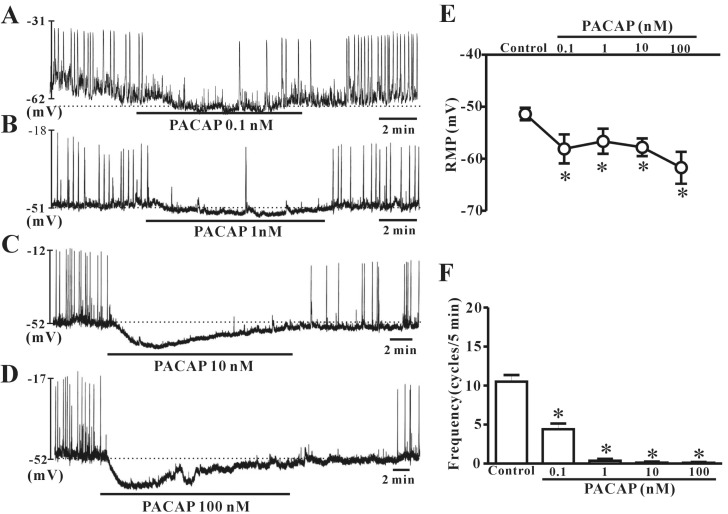
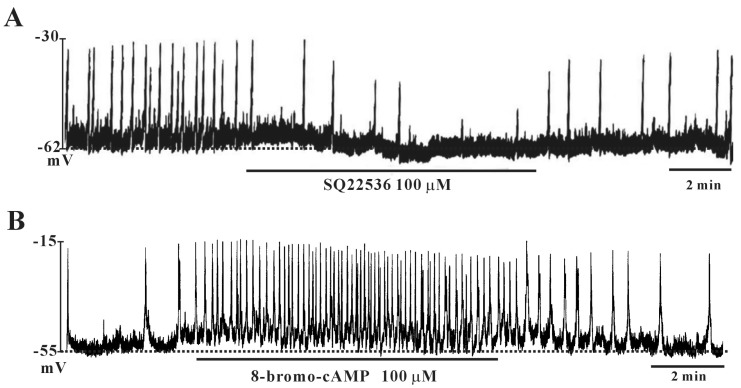
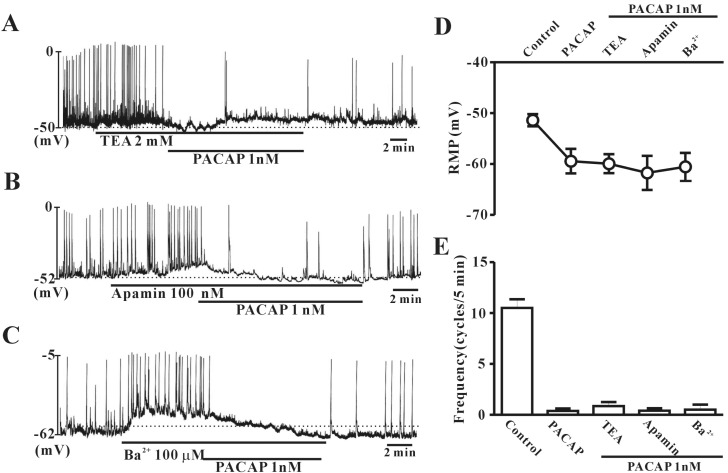
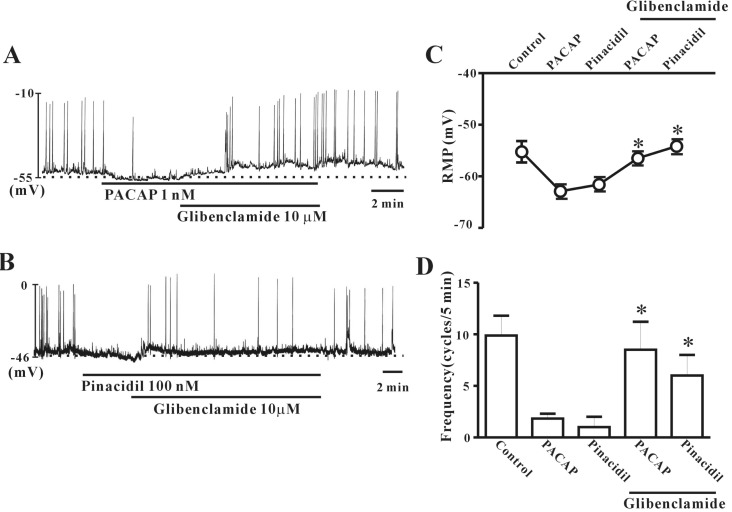
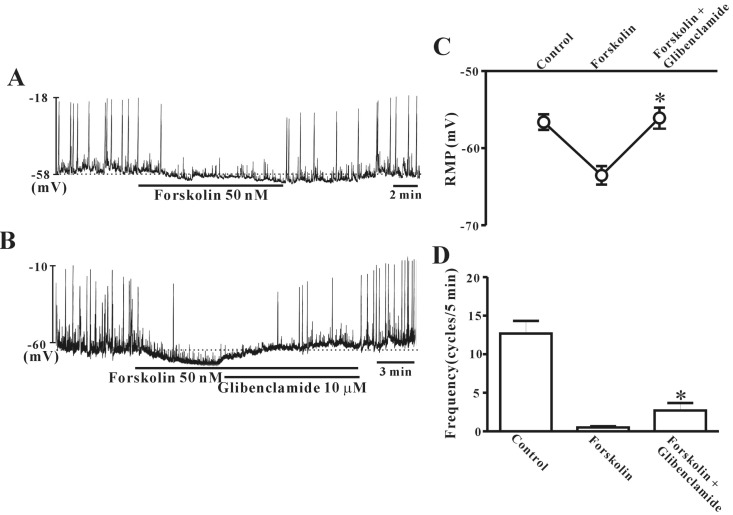
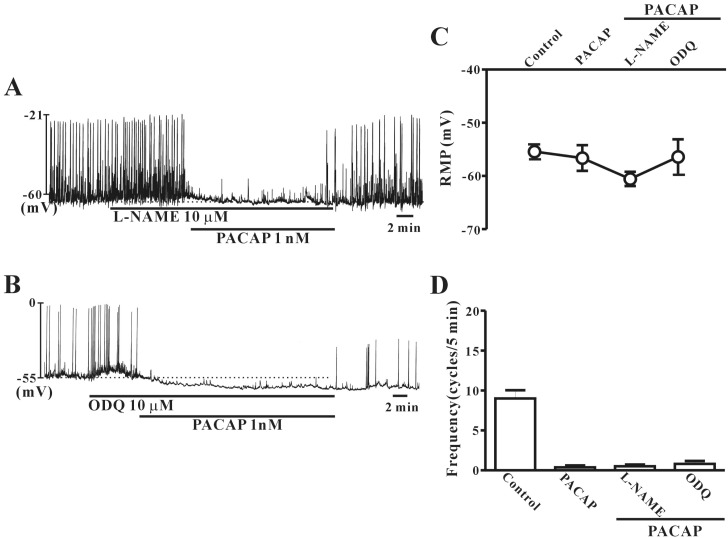
- This study aimed to investigate the effect of pituitary adenylate cyclase-activating peptide (PACAP) on the pacemaker activity of interstitial cells of Cajal (ICC) in mouse colon and to identify the underlying mechanisms of PACAP action. Spontaneous pacemaker activity of colonic ICC and the effects of PACAP were studied using electrophysiological recordings. Exogenously applied PACAP induced hyperpolarization of the cell membrane and inhibited pacemaker frequency in a dose-dependent manner (from 0.1 nM to 100 nM). To investigate cyclic AMP (cAMP) involvement in the effects of PACAP on ICC, SQ-22536 (an inhibitor of adenylate cyclase) and cell-permeable 8-bromo-cAMP were used. SQ-22536 decreased the frequency of pacemaker potentials, and cell-permeable 8-bromo-cAMP increased the frequency of pacemaker potentials. The effects of SQ-22536 on pacemaker potential frequency and membrane hyperpolarization were rescued by co-treatment with glibenclamide (an ATP-sensitive K+ channel blocker). However, neither N(G)-nitro-L-arginine methyl ester (L-NAME, a competitive inhibitor of NO synthase) nor 1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one (ODQ, an inhibitor of guanylate cyclase) had any effect on PACAP-induced activity. In conclusion, this study describes the effects of PACAP on ICC in the mouse colon. PACAP inhibited the pacemaker activity of ICC by acting through ATP-sensitive K+ channels. These results provide evidence of a physiological role for PACAP in regulating gastrointestinal (GI) motility through the modulation of ICC activity.
Keyword
MeSH Terms
-
8-Bromo Cyclic Adenosine Monophosphate
Animals
Cell Membrane
Colon*
Cyclic AMP
Glyburide
Interstitial Cells of Cajal*
Membranes
Mice
NG-Nitroarginine Methyl Ester
Pituitary Adenylate Cyclase-Activating Polypeptide*
8-Bromo Cyclic Adenosine Monophosphate
Cyclic AMP
Glyburide
NG-Nitroarginine Methyl Ester
Pituitary Adenylate Cyclase-Activating Polypeptide
Figure
Reference
-
1. Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000; 52:269–324. PMID: 10835102.2. Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000; 21:619–670. PMID: 11133067.
Article3. Portbury AL, McConalogue K, Furness JB, Young HM. Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res. 1995; 279:385–392. PMID: 7895276.
Article4. Katsoulis S, Clemens A, Schwörer H, Creutzfeldt W, Schmidt WE. PACAP is a stimulator of neurogenic contraction in guinea pig ileum. Am J Physiol. 1993; 265:G295–G302. PMID: 8368312.
Article5. Toyoshima M, Takeuchi T, Goto H, Mukai K, Shintani N, Hashimoto H, Baba A, Hata F. Roles of PACAP and PHI as inhibitory neurotransmitters in the circular muscle of mouse antrum. Pflugers Arch. 2006; 451:559–568. PMID: 16292577.
Article6. Mukai K, Takeuchi T, Toyoshima M, Satoh Y, Fujita A, Shintani N, Hashimoto H, Baba A, Hata F. PACAP- and PHI-mediated sustained relaxation in circular muscle of gastric fundus: findings obtained in PACAP knockout mice. Regul Pept. 2006; 133:54–61. PMID: 16229904.
Article7. Hagi K, Azuma YT, Nakajima H, Shintani N, Hashimoto H, Baba A, Takeuchi T. Involvements of PHI-nitric oxide and PACAP-BK channel in the sustained relaxation of mouse gastric fundus. Eur J Pharmacol. 2008; 590:80–86. PMID: 18602629.
Article8. Jin JG, Katsoulis S, Schmidt WE, Grider JR. Inhibitory transmission in tenia coli mediated by distinct vasoactive intestinal peptide and apamin-sensitive pituitary adenylate cyclase activating peptide receptors. J Pharmacol Exp Ther. 1994; 270:433–439. PMID: 8071835.9. McConalogue K, Lyster DJ, Furness JB. Electrophysiological analysis of the actions of pituitary adenylyl cyclase activating peptide in the taenia of the guinea-pig caecum. Naunyn Schmiedebergs Arch Pharmacol. 1995; 352:538–544. PMID: 8751083.
Article10. Katsoulis S, Schmidt WE, Schwarzhoff R, Folsch UR, Jin JG, Grider JR, Makhlouf GM. Inhibitory transmission in guinea pig stomach mediated by distinct receptors for pituitary adenylate cyclase-activating peptide. J Pharmacol Exp Ther. 1996; 278:199–204. PMID: 8764352.11. Ekblad E, Sundler F. Distinct receptors mediate pituitary adenylate cyclase-activating peptide- and vasoactive intestinal peptide-induced relaxation of rat ileal longitudinal muscle. Eur J Pharmacol. 1997; 334:61–66. PMID: 9346329.
Article12. Parkman HP, Pagano AP, Ryan JP. PACAP and VIP inhibit pyloric muscle through VIP/PACAP-preferring receptors. Regul Pept. 1997; 71:185–190. PMID: 9350977.
Article13. Grider JR, Katsoulis S, Schmidt WE, Jin JG. Regulation of the descending relaxation phase of intestinal peristalsis by PACAP. J Auton Nerv Syst. 1994; 50:151–159. PMID: 7884155.
Article14. Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995; 269:C1577–C1585. PMID: 8572188.
Article15. Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995; 373:347–349. PMID: 7530333.
Article16. So KY, Kim SH, Sohn HM, Choi SJ, Parajuli SP, Choi S, Yeum CH, Yoon PJ, Jun JY. Carbachol regulates pacemaker activities in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2009; 27:525–531. PMID: 19466600.
Article17. Shahi PK, Choi S, Zuo DC, Yeum CH, Yoon PJ, Lee J, Kim YD, Park CG, Kim MY, Shin HR, Oh HJ, Jun JY. 5-hydroxytryptamine generates tonic inward currents on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2011; 15:129–135. PMID: 21860590.
Article18. Park CG, Kim YD, Kim MY, Kim JS, Choi S, Yeum CH, Parajuli SP, Park JS, Jeong HS, So I, Kim KW, Jun JY. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007; 376:175–184. PMID: 17932655.19. Kishi M, Takeuchi T, Suthamnatpong N, Ishii T, Nishio H, Hata F, Takewaki T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br J Pharmacol. 1996; 119:623–630. PMID: 8904634.20. Mungun Z, Rossowski WJ, Coy DH. Pituitary adenylate cyclase activating polypeptide relxed gastrointestinal smooth muscles in rat. Clin Res. 1991; 39:236A.21. Katsoulis S, Clemens A, Schwörer H, Creutzfeldt W, Schmidt WE. Pituitary adenylate cyclase activating polypeptide (PACAP) is a potent relaxant of the rat ileum. Peptides. 1993; 14:587–592. PMID: 8101370.
Article22. Kim BJ, Lee JH, Jun JY, Chang IY, So I, Kim KW. Vasoactive intestinal polypeptide inhibits pacemaker activity via the nitric oxide-cGMP-protein kinase G pathway in the interstitial cells of Cajal of the murine small intestine. Mol Cells. 2006; 21:337–342. PMID: 16819295.23. Shuttleworth CW, Sanders KM. Involvement of nitric oxide in neuromuscular transmission in canine proximal colon. Proc Soc Exp Biol Med. 1996; 211:16–23. PMID: 8594614.
Article24. Jin JG, Murthy KS, Grider JR, Makhlouf GM. Activation of distinct cAMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am J Physiol. 1993; 264:G470–G477. PMID: 8384796.
Article25. McCulloch DA, MacKenzie CJ, Johnson MS, Robertson DN, Holland PJ, Ronaldson E, Lutz EM, Mitchell R. Additional signals from VPAC/PAC family receptors. Biochem Soc Trans. 2002; 30:441–446. PMID: 12196111.
Article26. Jun JY, Choi S, Yeum CH, Chang IY, Park CK, Kim MY, Kong ID, So I, Kim KW, You HJ. Noradrenaline inhibits pacemaker currents through stimulation of β1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine. Br J Pharmacol. 2004; 141:670–677. PMID: 14744802.27. Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006; 18:187–198. PMID: 17167224.
Article28. Bruch L, Bychkov R, Kästner A, Bülow T, Ried C, Gollasch M, Baumann G, Luft FC, Haller H. Pituitary adenylatecyclase-activating peptides relax human coronary arteries by activating K(ATP) and K(Ca) channels in smooth muscle cells. J Vasc Res. 1997; 34:11–18. PMID: 9075821.
Article29. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995; 268:C799–C822. PMID: 7733230.
Article30. Koh SD, Kim TW, Jun JY, Glasgow NJ, Ward SM, Sanders KM. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol. 2000; 527 Pt 1:149–162. PMID: 10944178.
Article31. Jun JY, Choi S, Chang IY, Yoon CK, Jeong HG, Kong ID, So I, Kim KW, You HJ. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005; 144:242–251. PMID: 15665863.32. Choi S, Park CG, Kim MY, Lim GH, Kim JH, Yeum CH, Yoon PJ, So I, Kim KW, Jun JY. Action of imipramine on activated ATP-sensitive K+ channels in interstitial cells of Cajal from murine small intestine. Life Sci. 2006; 78:2322–2328. PMID: 16266721.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Control Mechanisms of Ovulation by Pituitary Adenylate Cyclase-Activating Polypeptide
- Stimulation by EGF, bFGF and GnRH of Ovarian Pituitary Adenylate Cyclase-Activating Polypeptide Gene Expression in Cultured Rat Preovulatory Follicles
- Vasoactive Intestinal Peptide/Pituitary Adenylate Cyclase Activating Peptide Receptor Subtypes in Neuroblastoma, Stomach Cancer
- Control Mechanisms of Ovarian Follicle Development by Follicle Stimulating Hormone and Pituitary Adenylate Cyclase-activating Polypeptide
- Role of Pituitary Adenylate Cyclase Activating Polypeptide-(1-27) in the Clitorial Cavernosum Smooth Muscle