Korean J Physiol Pharmacol.
2014 Jun;18(3):269-278. 10.4196/kjpp.2014.18.3.269.
Lamotrigine Decreased Hippocampal Damage and Improved Vascular Risk Markers in a Rat Model of Pentylenetetrazole Induced Kindling Seizure
- Affiliations
-
- 1Department of Pharmacology, Faculty of Medicine, Ain Shams University, Cairo 11591, Egypt. helmy_amany@yahoo.com
- 2Department of Histology, Faculty of Medicine, Ain Shams University, Cairo 11591, Egypt.
- KMID: 2285520
- DOI: http://doi.org/10.4196/kjpp.2014.18.3.269
Abstract
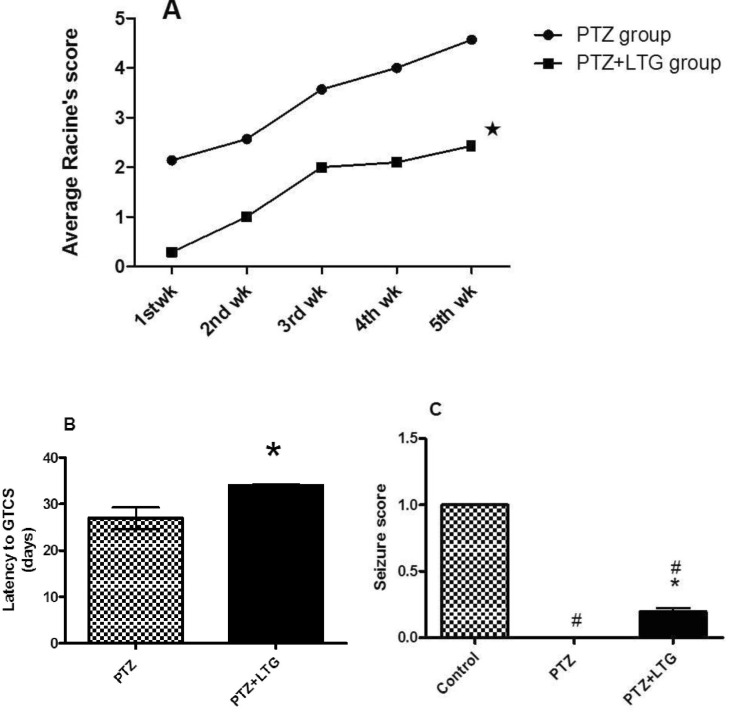
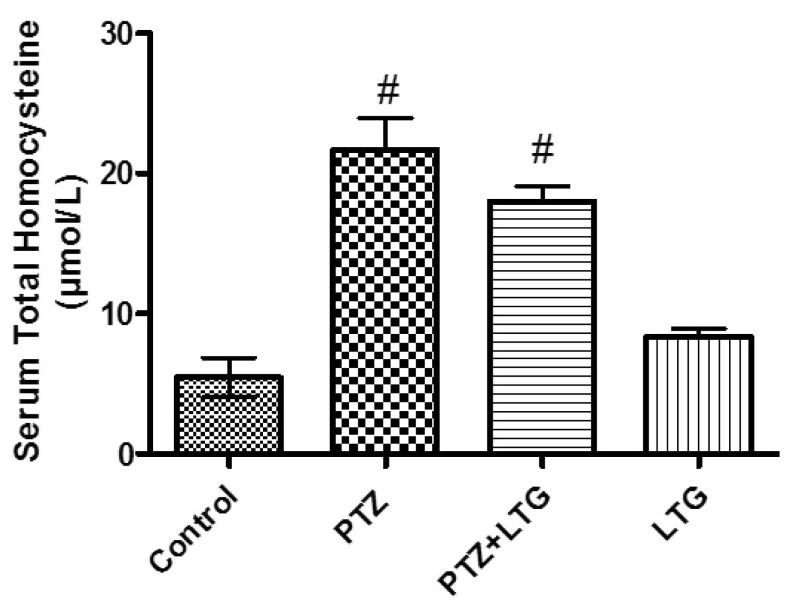
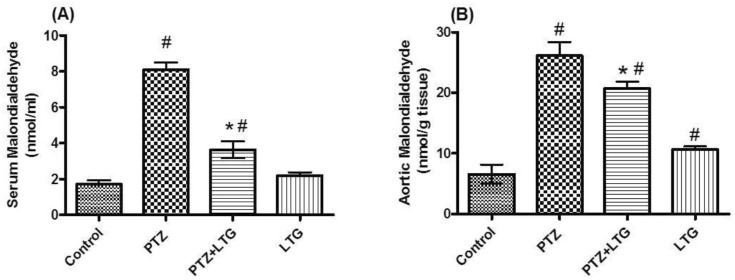
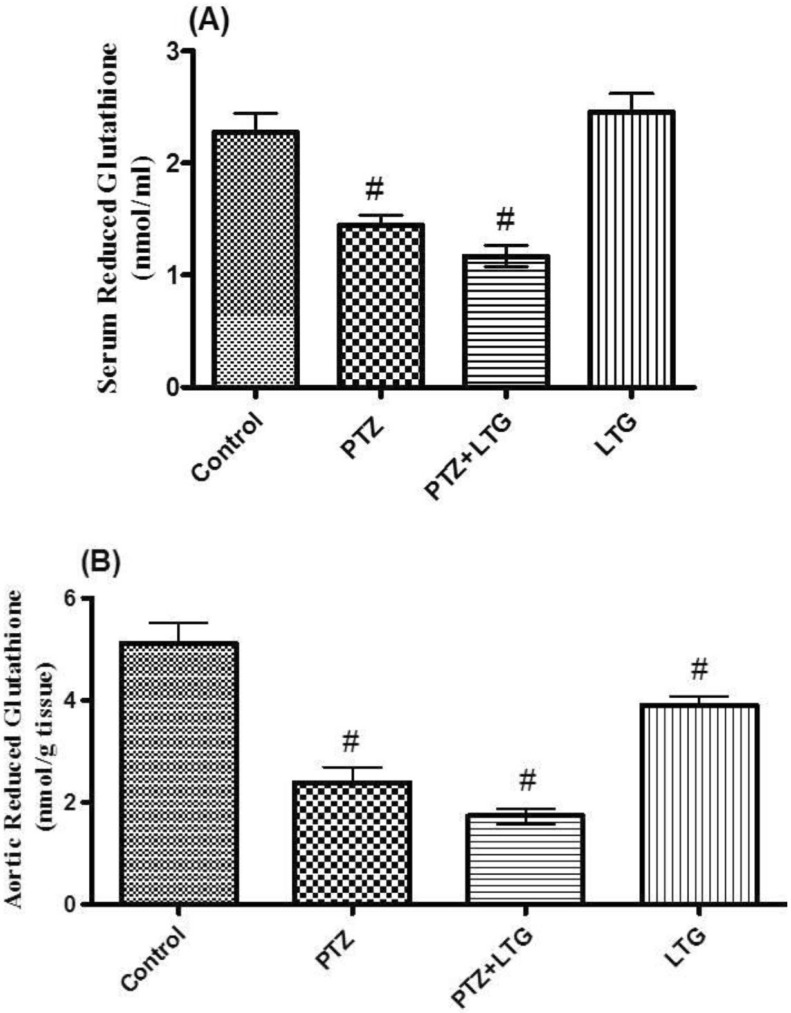
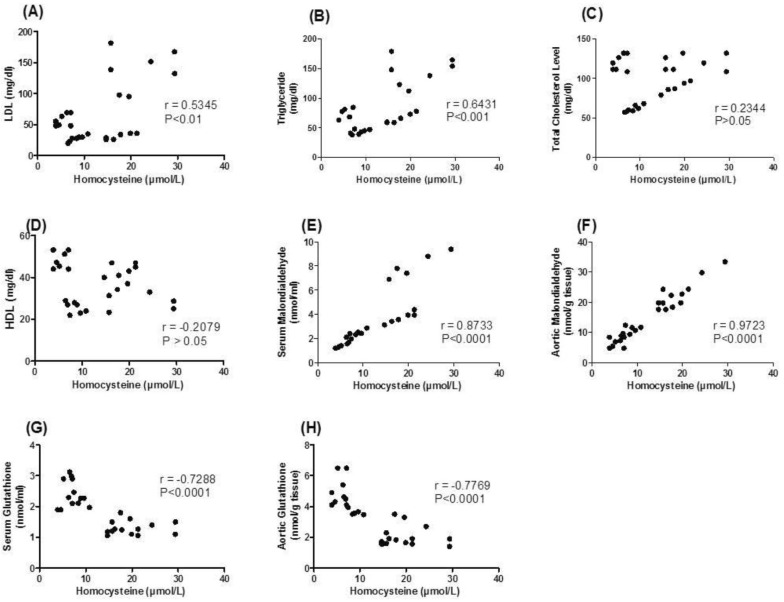
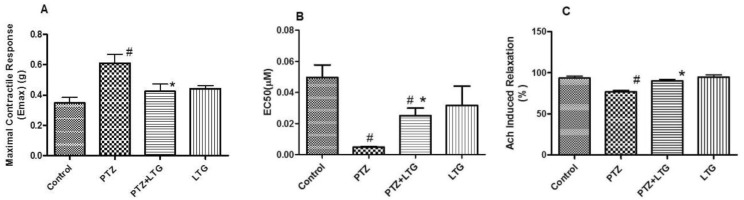
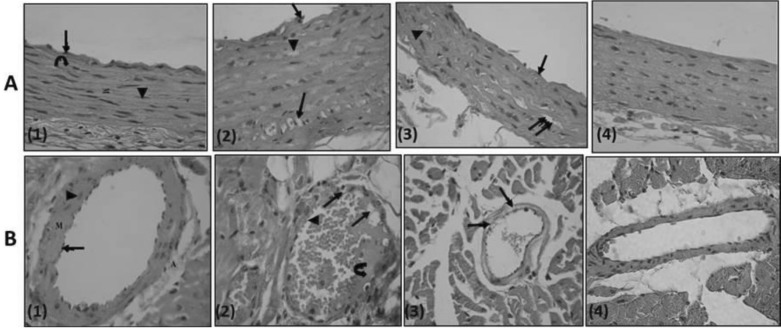
- Various antiepileptic drugs (AEDs) especially enzyme-inducing AEDs might be associated with increased vascular risk, through impairment of the endogenous antioxidative ability which may trigger oxygen-dependent tissue injury. Lamotrigine (LTG) a non-enzyme-inducing AED has scarce information regarding its effects on oxidative stress. The present study aimed to study the possible modulation of vascular risk factors of epileptogenesis by LTG, in a rat model of kindling seizure induced by pentylenetetrazole (PTZ). Four groups of male Wister rats were used; vehicle control group, PTZ group (alternate day PTZ, 30 mg/kg, i.p), LTG/PTZ group (LTG 20 mg/kg/day p.o and alternate day PTZ) and LTG group. The study period was 5 weeks. Lipoproteins and total homocysteine (tHcy), malondialdehyde (MDA) and reduced glutathione (GSH) were measured. Aortic endothelial function study and histopathological examination of the rats' brains, aortas and coronaries were conducted. Serum total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C), tHcy, MDA, GSH levels were significantly higher in epileptic rats than normal controls rats. A decrease in HDL-cholesterol with high atherosclerotic index was also demonstrated. The administration of LTG improved the PTZ-kindled seizures. It produced a significant decrease in TC, TG and LDL-cholesterol, MDA, aortic GSH and increase in HDL-cholesterol with no significant effect on serum GSH and tHcy levels. LTG improved endothelium-dependent relaxation, decreased hippocampal neurodegenerative changes and atherosclerotic changes of aortas and coronaries. LTG decreased seizures severity, hippocampal damage and improved vascular risk markers in this rat model of kindling seizures.
MeSH Terms
-
Animals
Anticonvulsants
Aorta
Brain
Cholesterol
Epilepsy
Glutathione
Homocysteine
Humans
Lipoproteins
Male
Malondialdehyde
Models, Animal*
Oxidative Stress
Pentylenetetrazole*
Rats
Relaxation
Risk Factors
Seizures*
Triglycerides
Anticonvulsants
Cholesterol
Glutathione
Homocysteine
Lipoproteins
Malondialdehyde
Pentylenetetrazole
Figure
Reference
-
1. Lopinto-Khoury C, Mintzer S. Antiepileptic drugs and markers of vascular risk. Curr Treat Options Neurol. 2010; 12:300–308. PMID: 20842589.
Article2. Nikolaos T, Stylianos G, Chryssoula N, Irini P, Christos M, Dimitrios T, Konstantinos P, Antonis T. The effect of long-term antiepileptic treatment on serum cholesterol (TC, HDL, LDL) and triglyceride levels in adult epileptic patients on monotherapy. Med Sci Monit. 2004; 10:MT50–MT52. PMID: 15039653.3. Attilakos A, Papakonstantinou E, Schulpis K, Voudris K, Katsarou E, Mastroyianni S, Garoufi A. Early effect of sodium valproate and carbamazepine monotherapy on homocysteine metabolism in children with epilepsy. Epilepsy Res. 2006; 71:229–232. PMID: 16889940.
Article4. Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001; 303:19–24. PMID: 11163018.
Article5. Tan TY, Lu CH, Chuang HY, Lin TK, Liou CW, Chang WN, Chuang YC. Long-term antiepileptic drug therapy contributes to the acceleration of atherosclerosis. Epilepsia. 2009; 50:1579–1586. PMID: 19292757.
Article6. Agarwal NB, Agarwal NK, Mediratta PK, Sharma KK. Effect of lamotrigine, oxcarbazepine and topiramate on cognitive functions and oxidative stress in PTZ-kindled mice. Seizure. 2011; 20:257–262. PMID: 21247777.
Article7. Martinc B, Grabnar I, Vovk T. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr Neuropharmacol. 2012; 10:328–343. PMID: 23730257.
Article8. Lipton SA, Kim WK, Choi YB, Kumar S, D'Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997; 94:5923–5928. PMID: 9159176.
Article9. Marangos PJ, Loftus T, Wiesner J, Lowe T, Rossi E, Browne CE, Gruber HE. Adenosinergic modulation of homocysteine-induced seizures in mice. Epilepsia. 1990; 31:239–246. PMID: 2344840.
Article10. Schwarz S, Zhou GZ. N-methyl-D-aspartate receptors and CNS symptoms of homocystinuria. Lancet. 1991; 337:1226–1227. PMID: 1673765.
Article11. Guilland JC, Favier A, Potier de Courcy G, Galan P, Hercberg S. Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease. Basic data. Pathol Biol (Paris). 2003; 51:101–110. PMID: 12801808.12. Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008; 83:1203–1212. PMID: 18990318.
Article13. Chuang YC, Chuang HY, Lin TK, Chang CC, Lu CH, Chang WN, Chen SD, Tan TY, Huang CR, Chan SH. Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia. 2012; 53:120–128. PMID: 22085257.
Article14. Cunningham MO, Jones RS. The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology. 2000; 39:2139–2146. PMID: 10963757.
Article15. Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004; 73:1–60. PMID: 15193778.
Article16. Mortazavi F, Ericson M, Story D, Hulce VD, Dunbar GL. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005; 7:629–638. PMID: 16246633.
Article17. Chen J, Quan QY, Yang F, Wang Y, Wang JC, Zhao G, Jiang W. Effects of lamotrigine and topiramate on hippocampal neurogenesis in experimental temporal-lobe epilepsy. Brain Res. 2010; 1313:270–282. PMID: 20025852.
Article18. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972; 32:281–294. PMID: 4110397.
Article19. Malhotra J, Gupta YK. Effect of adenosine receptor modulation on pentylenetetrazole-induced seizures in rats. Br J Pharmacol. 1997; 120:282–288. PMID: 9117121.
Article20. Schulpis K, Karikas GA. Serum cholesterol and triglyceride distribution in 7767 school-aged Greek children. Pediatrics. 1998; 101:861–864. PMID: 9565415.
Article21. Bhattacharya A, Smith GF, Cohen ML. Effect of LY287045, a thrombin/trypsin inhibitor, on thrombin and trypsin-induced aortic contraction and relaxation. J Pharmacol Exp Ther. 2001; 297:573–581. PMID: 11303045.22. Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. Oxford: Linacre House, Jordan Hill;1999. p. 94.23. Bancroft J, Gamble M. Theory and practice of histological techniques. 5th ed. London, Edinburgh, New York, Philadelphia, St. Louis, Sydney, Toronto: Churchill Livingstone;2002. p. 373.24. Jyoti A, Sethi P, Sharma D. Curcumin protects against electrobehavioral progression of seizures in the iron-induced experimental model of epileptogenesis. Epilepsy Behav. 2009; 14:300–308. PMID: 19100339.
Article25. Bharal N, Sahaya K, Jain S, Mediratta PK, Sharma KK. Curcumin has anticonvulsant activity on increasing current electroshock seizures in mice. Phytother Res. 2008; 22:1660–1664. PMID: 18661468.
Article26. Kumar G, Jones NC, Morris MJ, Rees S, O'Brien TJ, Salzberg MR. Early life stress enhancement of limbic epileptogenesis in adult rats: mechanistic insights. PLoS One. 2011; 6:e24033. PMID: 21957442.
Article27. Kullo IJ, Ballantyne CM. Conditional risk factors for atherosclerosis. Mayo Clin Proc. 2005; 80:219–230. PMID: 15704777.
Article28. Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, Sperling MR. Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann Neurol. 2009; 65:448–456. PMID: 19296463.
Article29. Unlüçerçi Y, Bekpinar S, Gürdöl F, Seferoğlu G. A study on the relationship between homocysteine and diabetic nephropathy in rats. Pharmacol Res. 2002; 45:249–252. PMID: 11884223.30. Sener U, Zorlu Y, Karaguzel O, Ozdamar O, Coker I, Topbas M. Effects of common anti-epileptic drug monotherapy on serum levels of homocysteine, vitamin B12, folic acid and vitamin B6. Seizure. 2006; 15:79–85. PMID: 16414291.
Article31. Gidal BE, Tamura T, Hammer A, Vuong A. Blood homocysteine, folate and vitamin B-12 concentrations in patients with epilepsy receiving lamotrigine or sodium valproate for initial monotherapy. Epilepsy Res. 2005; 64:161–166. PMID: 15936175.
Article32. Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001; 57:1422–1427. PMID: 11673583.
Article33. Ono H, Sakamoto A, Sakura N. Plasma total glutathione concentrations in epileptic patients taking anticonvulsants. Clin Chim Acta. 2000; 298:135–143. PMID: 10876010.
Article34. Gupta YK, Malhotra J. Antiepileptic drug therapy in the twenty first century. Indian J Physiol Pharmacol. 2000; 44:8–23. PMID: 10919091.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Persistence of Kindling Phenomenon after Injections of Pentylenetetrazol in Rats
- Expression of c-fos mRNA in the Hippocampus of Pentylenetetrazol Kindling Rat
- The Immunohistochemical Expression of Neuronal Nitric Oxide Synthase in Rat Hippocampus after Pentylenetetrazole-Induced Seizures
- Mossy Fiber Synaptic Reorganization of Dentate Gyrus by Pentylenetetrazol Kindling Rats
- Expression of c-myc mRNA in the Hippocampus of Pentylenetetrazol Kindling Rat