Korean J Physiol Pharmacol.
2010 Aug;14(4):199-204. 10.4196/kjpp.2010.14.4.199.
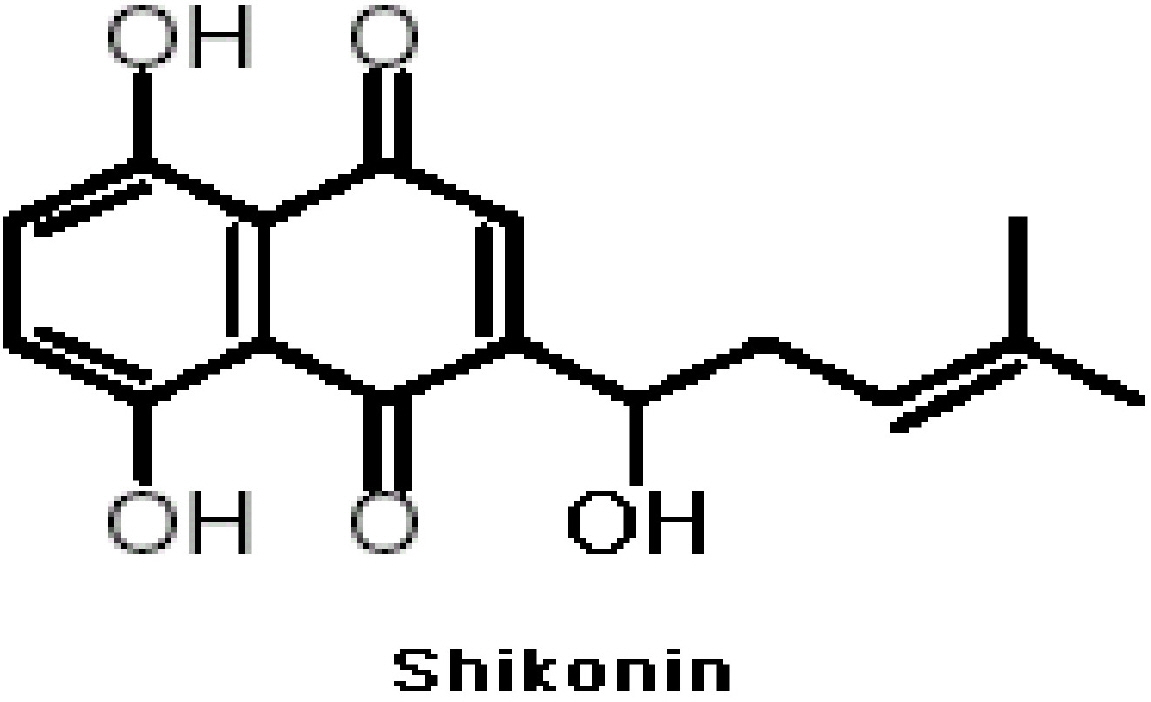
The Efficacy of Shikonin on Cartilage Protection in a Mouse Model of Rheumatoid Arthritis
- Affiliations
-
- 1Department of Herbal Crop Research, National Institute of Horticultural & Herbal Science, Rural Development Administration, Eumseong 369-873, Korea.
- 2Department of Rheumatology, School of Medicine, Kyung Hee University, Seoul 130-701, Korea.
- 3Department of Clinical Pharmacology, School of Medicine, Kyung Hee University, Seoul 130-701, Korea. ysvin@khu.ac.kr
- KMID: 2285403
- DOI: http://doi.org/10.4196/kjpp.2010.14.4.199
Abstract
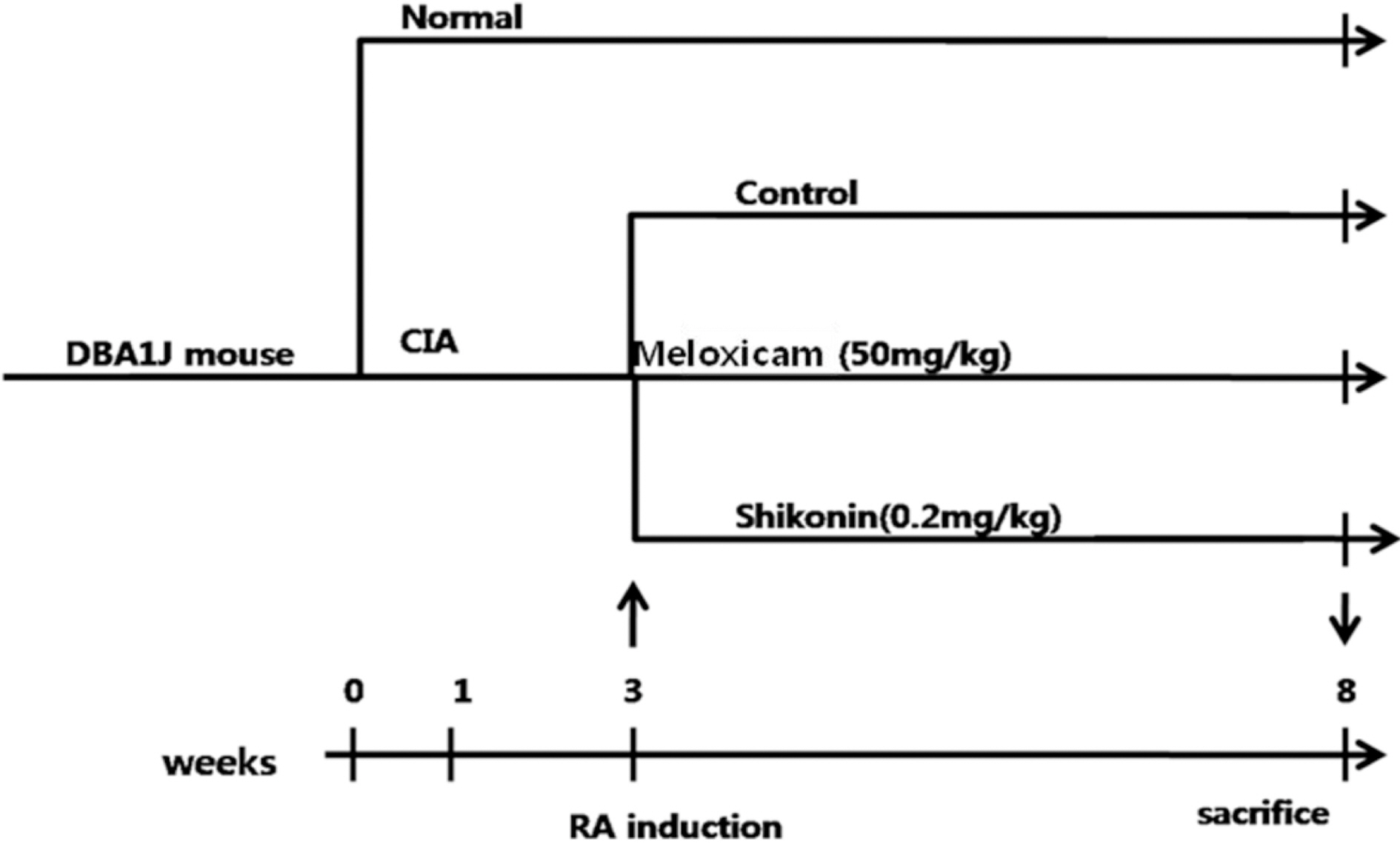
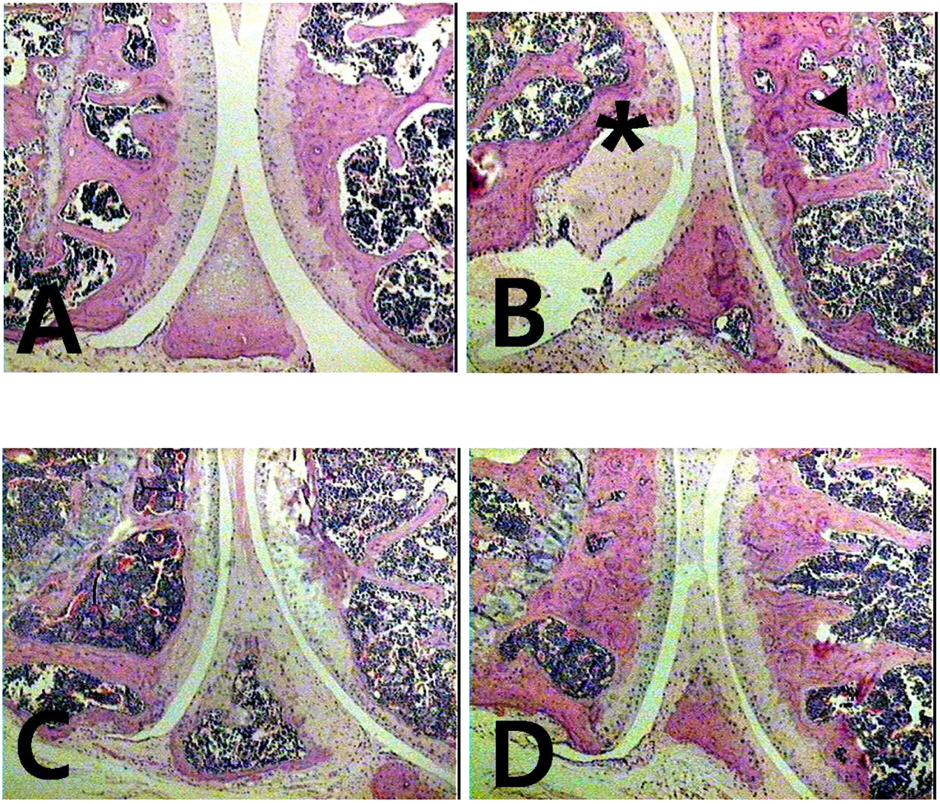
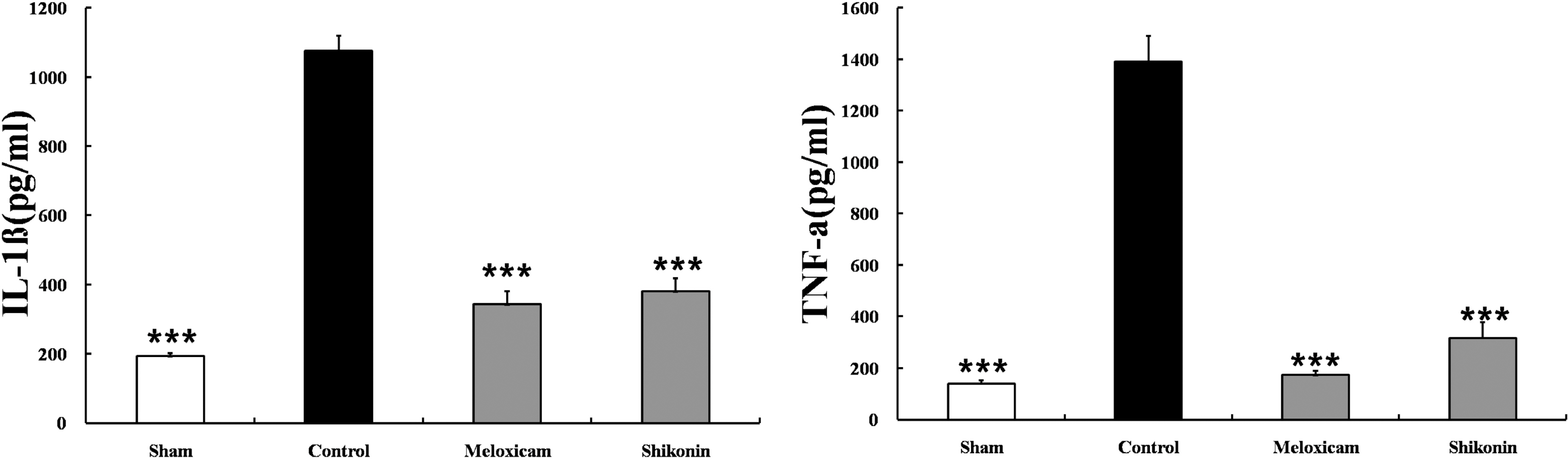
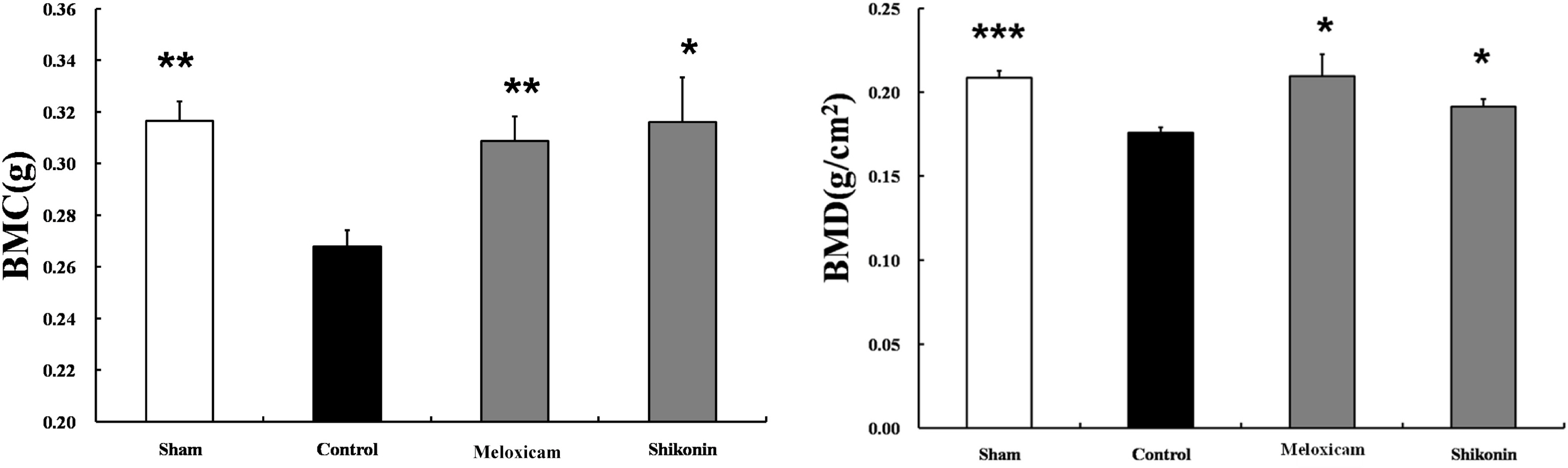
- The potential therapeutic action of shikonin in an experimental model of rheumatoid arthritis (RA) was investigated. As a RA animal model, DBA/1J mice were immunized two times with type II collagen. After the second collagen immunization, mice were orally administered shikonin (2 mg/kg) once a day for 35 days, and the incidence, clinical score, bone mineral density (BMD), bone mineral content (BMC) and joint histopathology were evaluated. BMD in the proximal regions of the tibia largely increased in the shikonin treatment group compared with the control group. We also examined the effect of shikonin on inflammatory cytokines and cartilage protection. Shikonin treatment significantly reduced the incidence and severity of collagen-induced arthritis (CIA), markedly abrogating joint swelling and cartilage destruction. Shikonin also significantly inhibited the production of matrix metalloproteinase (MMP)-1 and up-regulated tissue inhibitors of metalloproteinase (TIMP)-1 in mice with CIA. In conclusion, shikonin exerted therapeutic effects through regulation of MMP/TIMP; these results suggest that shikonin is an outstanding candidate as a cartilage protective medicine for RA.
Keyword
MeSH Terms
-
Animals
Arthritis, Experimental
Arthritis, Rheumatoid
Bone Density
Cartilage
Collagen
Collagen Type II
Cytokines
Immunization
Incidence
Joints
Mice
Models, Animal
Models, Theoretical
Naphthoquinones
Tibia
Tissue Inhibitor of Metalloproteinase-1
Collagen
Collagen Type II
Cytokines
Naphthoquinones
Tissue Inhibitor of Metalloproteinase-1
Figure
Cited by 1 articles
-
Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
Tingyu Wang, Jun Li, Zhigui Jin, Feihua Wu, Yunwu Li, Xiaohua Wang, Hong Zhou, Qian Zhou
Korean J Physiol Pharmacol. 2015;19(2):83-88. doi: 10.4196/kjpp.2015.19.2.83.
Reference
-
References
1. Hirayama T, Danks L, Sabokbar A, Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford). 2002; 41:1232–1239.
Article2. Park YR, Eun JS, Choi HJ, Nepal M, Kim DK, Seo SY, Rihua LI, Moon WS, Cho NP, Cho SD, Bae TS, Kim BI, Soh YJ. Hexane-soluble fraction of the common Fig, Ficus carica, inhibits osteoclast differentiation in murine bone marrow-derived macrophages and raw 264.7. Korean J Physiol Pharmacol. 2009; 13:417–424.3. Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NF kappa B ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002; 30:340–346.4. Garnero P, Geusens P, Landewe R. Biochemical markers of joint tissue turnover in early rheumatoid arthritis. Clin Exp Rheumatol. 2003; 21:S54–S58.5. Klimiuk PA, Sierakowski S, Domyslawska I, Chwiecko J. Effect of repeated infliximab therapy on serum matrix metalloproteinases and tissue inhibitors of metalloproteinases and tissue inhibitors of metalloproteinases in patients with rheumatoid arthritis. J Rheumatol. 2004; 31:238–242.6. Matrisian L. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990; 6:121–125.
Article7. Goldring SR, Gravallese EM. Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol. 2000; 12:195–199.
Article8. Jones SA, Kennedy AJ, Roberts NA. Assessment of drugs for activity in established type II collagen arthritis. Agents Actions. 1982; 12:650–656.
Article9. Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007; 2:1269–1275.
Article10. Hur GM, Hwang YB, Lee JH, Bae SH, Park JS, Lee CJ, Seok JH. Caffeic acid phenethyl ester inhibits the PKC-induced IL-6 gene expression in the synoviocytes of rheumatoid arthritis patients. Korean J Physiol Pharmacol. 2003; 7:363–368.11. Imaizumi K, Hinoue H, Ueno M, Takata I, Sato T, Minato Y, Takeshita M, Okaniwa A. Histopathological study of arthritic lesions induced by immunization with type II collagen in DBA/1J mouse. Jikken Dobutsu. 1990; 39:27–34.
Article12. Jin R, Wan LL, Mitsuishi T, Kodama K, Kurashige S. Immunomodulative effects of Chinese herbs in mice treated with anti-tumor agent cyclophosphamide. Yakugaku. Zasshi. 1994; 114:533–538.13. Chung HS, Kang M, Cho C, Park S, Kim H, Yoon YS, Kang J, Shin MK, Hong MC, Bae H. Inhibition of lipopolysaccharide and interferon-gamma-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by Lithospermi radix in mouse peritoneal macrophages. J Ethnopharmacol. 2005; 102:412–417.
Article14. Jin R, Kurashige S. Effects of Chinese herbs on macrophage functions in N-butyl-N-butanolnitrosoamine treated mice. Immunopharmacol. Immunotoxicol. 1996; 18:105–114.15. Lee HG. Bone Cho Kang Mook [compendium of material medica]. Seoul: Yeoil. 2007. (in Korean).16. Vassilios PP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int. 1999; 38:270–301.17. Ishida T, Sakaguchi I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biol Pharm Bull. 2007; 30:928–934.
Article18. Ahmed M, Khanna D, Furst DE. Meloxicam in the rheumatoid arthritis. Expert Opin Drug Metab Toxicol. 2005; 1:739–751.19. Campagnuolo G, Bolon B, Feige U. Kinetics of bone protection by recombinant osteoprotegerin therapy in Lewis rats with adjuvant arthritis. Arthritis Rheum. 2002; 46:1926–1936.
Article20. Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003; 18:1206–1216.
Article21. Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993; 94:145–149.
Article22. Chikanza IC, Kingsley G, Panayi GS. Peripheral blood and synovial fluid monocyte expression of interleukin 1α and TNF-α during active rheumatoid arthritis. J Rheumatol. 1995; 22:600–606.23. Woessner JF. Matrix metalloproteinase and their inhibitors in connective tissue remodeling. FASEB J. 1998; 5:2145–2154.24. Jackson CJ, Arkell J, Nguyen M. Rheumatoids synovial endothelial cells secrete decreased levels of tissue inhibitor of MMP (TIMP1). Ann Rheum Dis. 1998; 57:158–161.25. Green MJ, Gough AK, Devlin J, Smith J, Astin P, Taylor D. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology. 2003; 42:82–88.
Article26. Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC, Huizinga TW. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002; 61:975–980.27. Enokida M, Yamasaki D, Okano T, Hagino H, Morio Y, Teshima R. Bone mass changes of tibial and vertebral bones in young and adult rats with collagen-induced arthritis. Bone. 2001; 28:87–93.
Article28. Minne HW, Pfeilschifter J, Scharla S, Mutschelknauss S, Schwarz A, Krempien B, Ziegler R. Inflammation-mediated osteopenia in the rat: a new animal model for pathological loss of bone mass. Endocrinology. 1984; 115:50–54.
Article29. Choi S, Lee YA, Hong SJ, Lee GJ, Kang SW, Park JH, Park JH, Park HK. Evaluation of inflammatory change and bone erosion using a murine type II collagen-induced arthritis model. Rheumatol Int. 2010. [Epub ahead of print].
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Shikonin ameliorates salivary gland damage and inflammation in a mouse model of Sjögren’s syndrome by modulating MAPK signaling pathway
- Experimental Animal Models for Rheumatoid Arthritis: Methods and Applications
- The use of animal models in rheumatoid arthritis research
- A New Paradigm for Pathogenesis of Rheumatoid Arthritis
- Cytokines in rheumatoid arthritis