J Adv Prosthodont.
2014 Oct;6(5):351-360. 10.4047/jap.2014.6.5.351.
Characteristics and response of mouse bone marrow derived novel low adherent mesenchymal stem cells acquired by quantification of extracellular matrix
- Affiliations
-
- 1Department of Prosthodontics & Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Republic of Korea.
- 2Department of Prosthodontics & Dental Research Institute, Seoul National University Dental Hospital, School of Dentistry, Seoul National University, Seoul, Republic of Korea. ksy0617@snu.ac.kr
- 3Department of Prosthodontics, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Republic of Korea.
- 4Department of Prosthodontics, Ewha Womans University, Seoul, Republic of Korea.
- KMID: 2284735
- DOI: http://doi.org/10.4047/jap.2014.6.5.351
Abstract
- PURPOSE
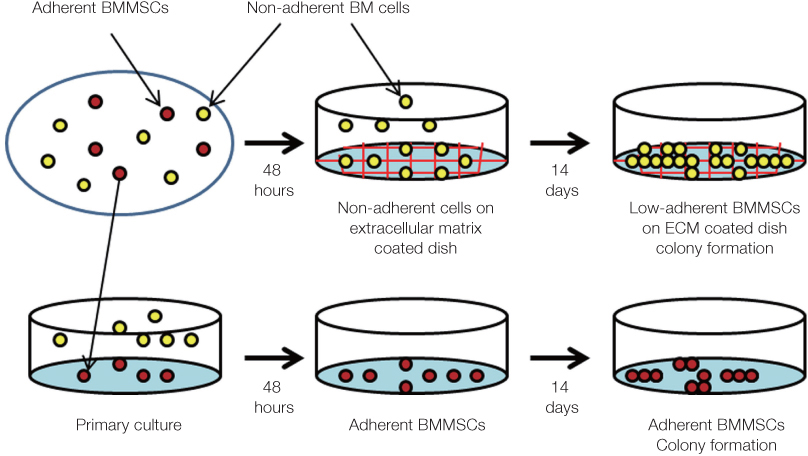
The aim of present study was to identify characteristic and response of mouse bone marrow (BM) derived low-adherent bone marrow mesenchymal stem cells (BMMSCs) obtained by quantification of extracellular matrix (ECM).
MATERIALS AND METHODS
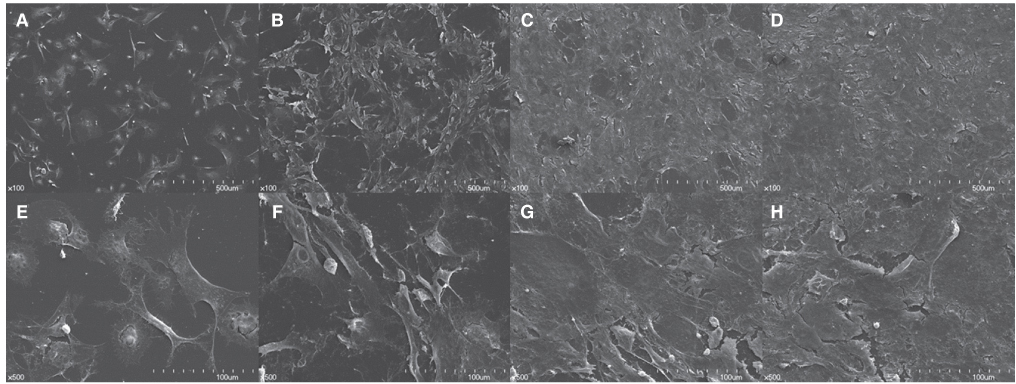
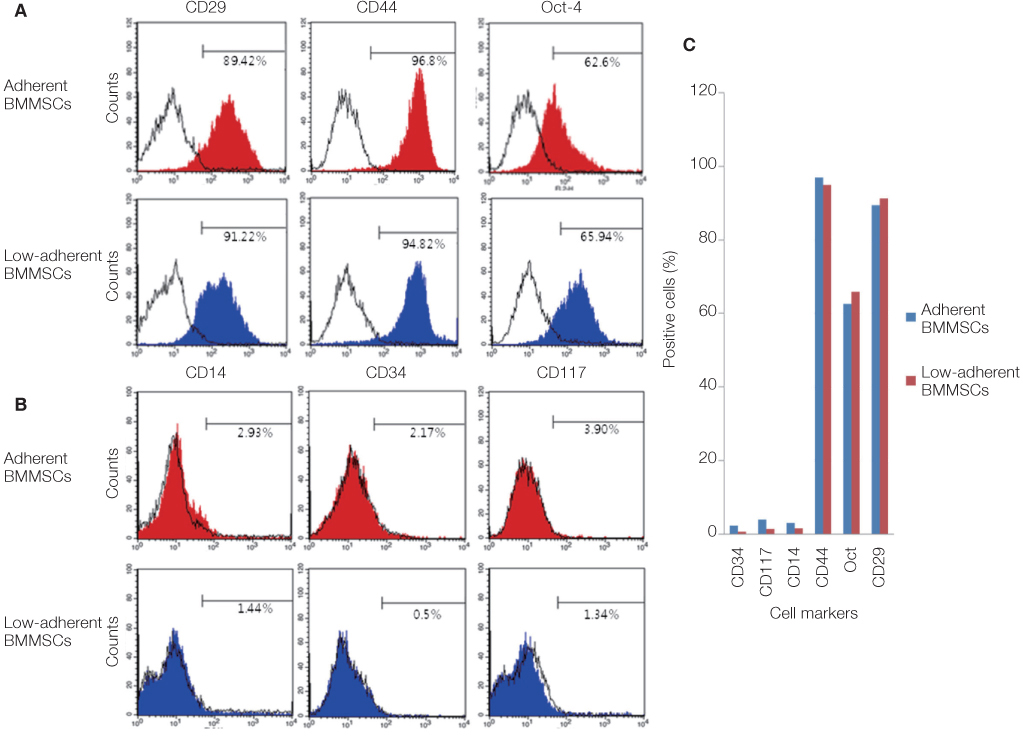
Non-adherent cells acquired by ECM coated dishes were termed low-adherent BMMSCs and these cells were analyzed by in vitro and in vivo methods, including colony forming unit fibroblast (CFU-f), bromodeoxyuridine (BrdU), multi-potential differentiation, flow cytometry and transplantation into nude mouse to measure the bone formation ability of these low-adherent BMMSCs. Titanium (Ti) discs with machined and anodized surfaces were prepared. Adherent and low-adherent BMMSCs were cultured on the Ti discs for testing their proliferation.
RESULTS
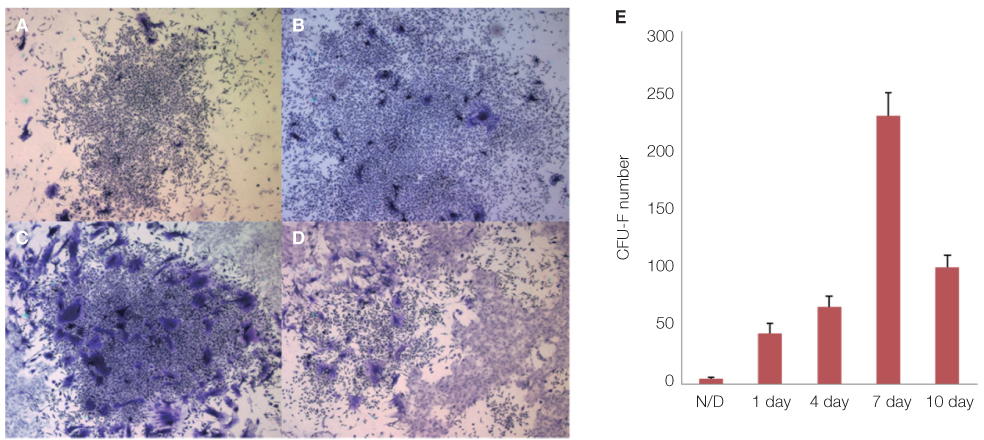
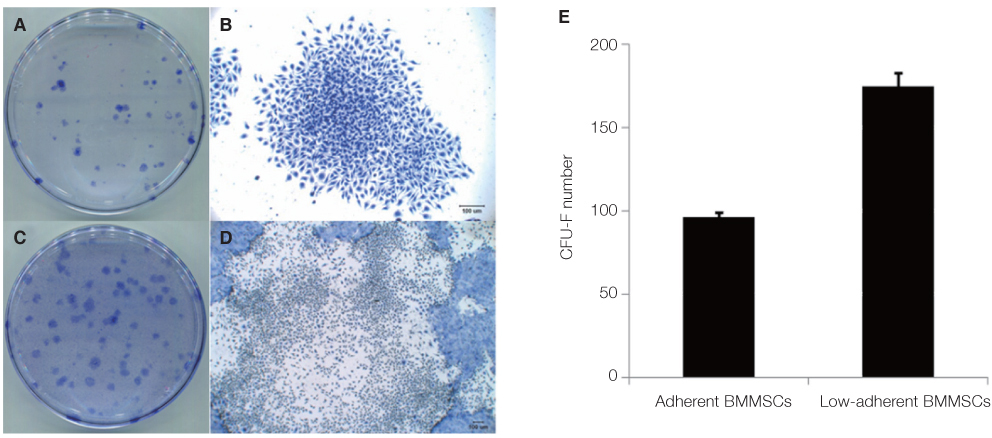
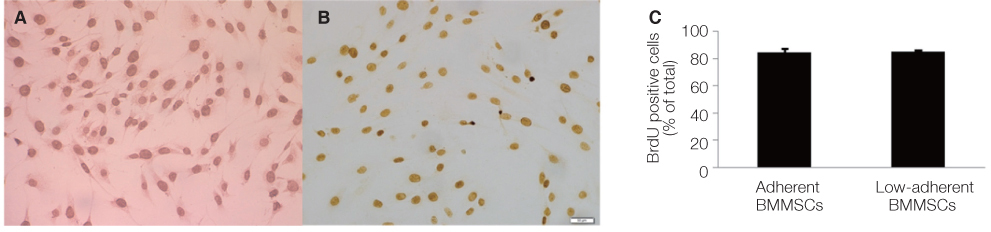
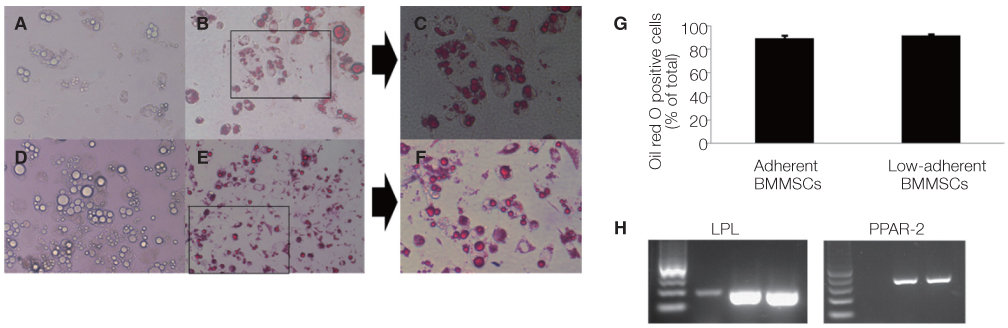
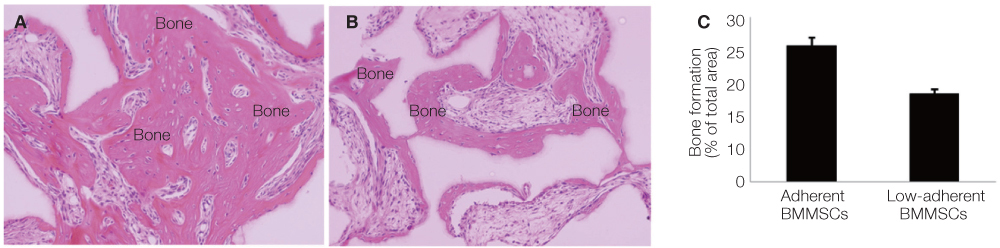
The amount of CFU-f cells was significantly higher when non-adherent cells were cultured on ECM coated dishes, which was made by 7 days culturing of adherent BMMSCs. Low-adherent BMMSCs had proliferation and differentiation potential as adherent BMMSCs in vitro. The mean amount bone formation of adherent and low-adherent BMMSCs was also investigated in vivo. There was higher cell proliferation appearance in adherent and low-adherent BMMSCs seeded on anodized Ti discs than machined Ti discs by time.
CONCLUSION
Low-adherent BMMSCs acquired by ECM from non-adherent cell populations maintained potential characteristic similar to those of the adherent BMMSCs and therefore could be used effectively as adherent BMMSCs in clinic.
Keyword
MeSH Terms
Figure
Reference
-
1. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.2. Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004; 22:823–831.3. Blazsek I, Chagraoui J, Péault B. Ontogenic emergence of the hematon, a morphogenetic stromal unit that supports multipotential hematopoietic progenitors in mouse bone marrow. Blood. 2000; 96:3763–3771.4. Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008; 36:1176–1185.5. Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001; 98:2615–2625.6. Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002; 170:73–82.7. Deschaseaux F, Gindraux F, Saadi R, Obert L, Chalmers D, Herve P. Direct selection of human bone marrow mesenchymal stem cells using an anti-CD49a antibody reveals their CD45med,low phenotype. Br J Haematol. 2003; 122:506–517.8. Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005; 23:1105–1112.9. Tondreau T, Lagneaux L, Dejeneffe M, Delforge A, Massy M, Mortier C, Bron D. Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: phenotype, proliferation kinetics and differentiation potential. Cytotherapy. 2004; 6:372–379.10. Clarke E, McCann SR. Stromal colonies can be grown from the non-adherent cells in human long-term bone marrow cultures. Eur J Haematol. 1991; 46:296–300.11. Falla N, Van Vlasselaer , Bierkens J, Borremans B, Schoeters G, Van Gorp U. Characterization of a 5-fluorouracil-enriched osteoprogenitor population of the murine bone marrow. Blood. 1993; 82:3580–3591.12. Long MW, Williams JL, Mann KG. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest. 1990; 86:1387–1395.13. Scutt A, Zeschnigk M, Bertram P. PGE2 induces the transition from non-adherent to adherent bone marrow mesenchymal precursor cells via a cAMP/EP2-mediated mechanism. Prostaglandins. 1995; 49:383–395.14. Włodarski KH, Galus R, Włodarski P. Non-adherent bone marrow cells are a rich source of cells forming bone in vivo. Folia Biol (Praha). 2004; 50:167–173.15. Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006; 37:967–976.16. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999; 5:434–438.17. Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006; 24:2202–2208.18. Mansilla E, Marín GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzmán A, Nuñez A, Gil MA, Piccinelli G, Ibar R, Soratti C. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006; 38:967–969.19. Akiyama K, You YO, Yamaza T, Chen C, Tang L, Jin Y, Chen XD, Gronthos S, Shi S. Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Res Ther. 2012; 3:40.20. Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007; 22:1943–1956.21. Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4:102–106.22. Park KH, Koak JY, Kim SK, Heo SJ. Wettability and cellular response of UV light irradiated anodized titanium surface. J Adv Prosthodont. 2011; 3:63–68.23. Park KH, Koak JY, Kim SK, Han CH, Heo SJ. The effect of ultraviolet-C irradiation via a bactericidal ultraviolet sterilizer on an anodized titanium implant: a study in rabbits. Int J Oral Maxillofac Implants. 2013; 28:57–66.24. Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974; 2:83–92.25. Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008; 68:4229–4238.26. Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010; 19:1095–1107.27. Long MW, Robinson JA, Ashcraft EA, Mann KG. Regulation of human bone marrow-derived osteoprogenitor cells by osteogenic growth factors. J Clin Invest. 1995; 95:881–887.28. Oyajobi BO, Lomri A, Hott M, Marie PJ. Isolation and characterization of human clonogenic osteoblast progenitors immunoselected from fetal bone marrow stroma using STRO-1 monoclonal antibody. J Bone Miner Res. 1999; 14:351–361.29. Baksh D, Davies JE, Zandstra PW. Adult human bone marrow-derived mesenchymal progenitor cells are capable of adhesion-independent survival and expansion. Exp Hematol. 2003; 31:723–732.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Mesenchymal Stem Cells for Tissue Engineering and Regenerative Medicine