Diabetes Metab J.
2014 Oct;38(5):337-345. 10.4093/dmj.2014.38.5.337.
Diabetic Cardiomyopathy and Its Prevention by Nrf2: Current Status
- Affiliations
-
- 1Kosair Children's Hospital Research Institute, Department of Pediatrics, the University of Louisville School of Medicine, Louisville, KY, USA. L0cai001@louisville.edu
- 2The Center of Cardiovascular Diseases, the First Hospital of Jilin University, Changchun, China.
- KMID: 2280677
- DOI: http://doi.org/10.4093/dmj.2014.38.5.337
Abstract
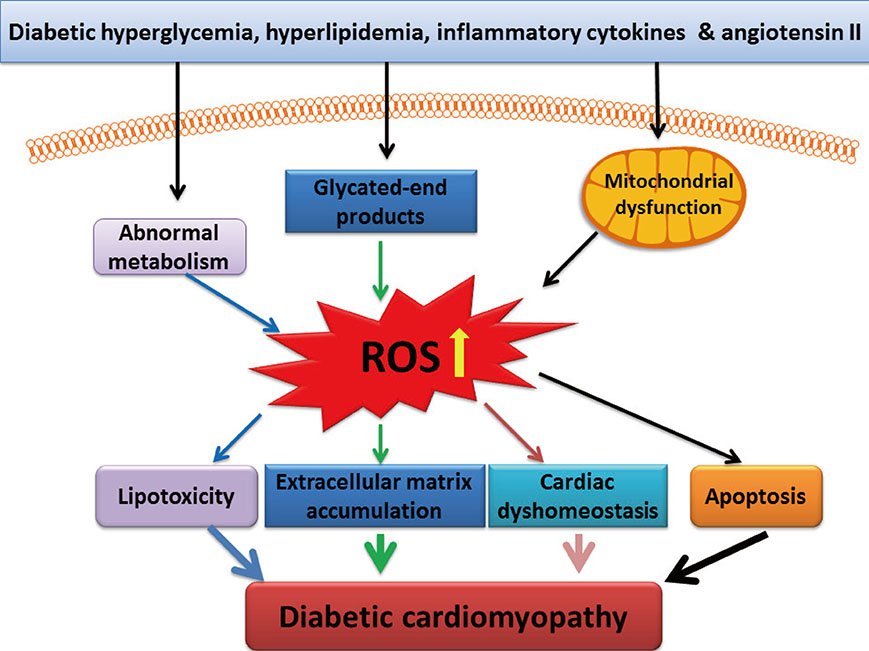
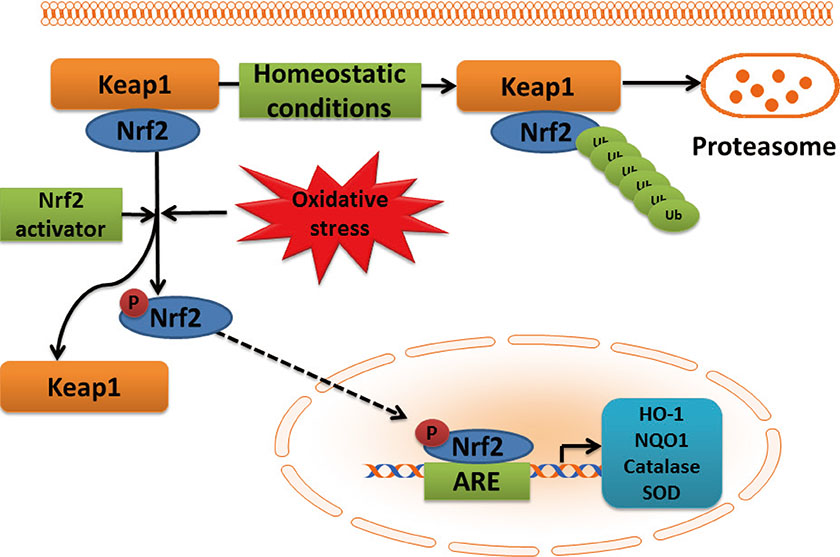
- Diabetic cardiomyopathy (DCM), as one of the major cardiac complications in diabetic patients, is known to related with oxidative stress that is due to a severe imbalance between reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) generation and their clearance by antioxidant defense systems. Transcription factor nuclear factor NF-E2-related factor 2 (Nrf2) plays an important role in maintaining the oxidative homeostasis by regulating multiple downstream antioxidants. Diabetes may up-regulate several antioxidants in the heart as a compensative mechanism at early stage, but at late stage, diabetes not only generates extra ROS and/or RNS but also impairs antioxidant capacity in the heart, including Nrf2. In an early study, we have established that Nrf2 protect the cardiac cells and heart from high level of glucose in vitro and hyperglycemia in vivo, and in the following study demonstrated the significant down-regulation of cardiac Nrf2 expression in diabetic animals and patients. Using Nrf2-KO mice or Nrf2 inducers, blooming evidence has indicated the important protection by Nrf2 from cardiac pathogenesis in the diabetes. Therefore, this brief review summarizes the status of studies on Nrf2's role in preventing DCM and even other complications, the need for new and safe Nrf2 inducer screening and the precaution for the undesirable side of Nrf2 under certain conditions.
MeSH Terms
-
Animals
Antioxidants
Diabetic Cardiomyopathies*
Down-Regulation
Glucose
Heart
Homeostasis
Humans
Hyperglycemia
Mass Screening
Mice
NF-E2-Related Factor 2
Oxidative Stress
Reactive Nitrogen Species
Reactive Oxygen Species
Transcription Factors
Antioxidants
Glucose
NF-E2-Related Factor 2
Reactive Nitrogen Species
Reactive Oxygen Species
Transcription Factors
Figure
Reference
-
1. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007; 115:3213–3223.2. Monkemann H, De Vriese AS, Blom HJ, Kluijtmans LA, Heil SG, Schild HH, Golubnitschaja O. Early molecular events in the development of the diabetic cardiomyopathy. Amino Acids. 2002; 23:331–336.3. Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001; 1:181–193.4. Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF. Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol. 2007; 42:884–895.5. Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006; 48:1688–1697.6. Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem. 2004; 261:187–191.7. Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980; 65:128–135.8. Chen Y, Saari JT, Kang YJ. Weak antioxidant defenses make the heart a target for damage in copper-deficient rats. Free Radic Biol Med. 1994; 17:529–536.9. Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, Rossi F, D'Amico M. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005; 54:803–810.10. Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003; 52:2338–2345.11. Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005; 54:778–784.12. Koh KK, Oh PC, Quon MJ. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res. 2009; 81:649–659.13. Giannini C, Lombardo F, Curro F, Pomilio M, Bucciarelli T, Chiarelli F, Mohn A. Effects of high-dose vitamin E supplementation on oxidative stress and microalbuminuria in young adult patients with childhood onset type 1 diabetes mellitus. Diabetes Metab Res Rev. 2007; 23:539–546.14. Ceriello A. New insights on oxidative stress and diabetic complications may lead to a "causal" antioxidant therapy. Diabetes Care. 2003; 26:1589–1596.15. Cai L. Diabetic cardiomyopathy and its prevention by metallothionein: experimental evidence, possible mechanisms and clinical implications. Curr Med Chem. 2007; 14:2193–2203.16. Cai L, Klein JB, Kang YJ. Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage. J Biol Chem. 2000; 275:38957–38960.17. Quesada AR, Byrnes RW, Krezoski SO, Petering DH. Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites. Arch Biochem Biophys. 1996; 334:241–250.18. Thornalley PJ, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985; 827:36–44.19. Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005; 54:1829–1837.20. Zhou G, Li X, Hein DW, Xiang X, Marshall JP, Prabhu SD, Cai L. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008; 52:655–666.21. Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med. 2009; 13(8A):1499–1512.22. Wang Y, Feng W, Xue W, Tan Y, Hein DW, Li XK, Cai L. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes. 2009; 58:1391–1402.23. Cai L. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med. 2006; 41:851–861.24. Yang L, Li H, Yu T, Zhao H, Cherian MG, Cai L, Liu Y. Polymorphisms in metallothionein-1 and -2 genes associated with the risk of type 2 diabetes mellitus and its complications. Am J Physiol Endocrinol Metab. 2008; 294:E987–E992.25. Li J, Ichikawa T, Janicki JS, Cui T. Targeting the Nrf2 pathway against cardiovascular disease. Expert Opin Ther Targets. 2009; 13:785–794.26. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009; 284:13291–13295.27. He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009; 46:47–58.28. Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, Cui T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011; 60:625–633.29. Cheng X, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin X, Mayr M, Siow RC, Mann GE. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013; 62:4088–4097.30. Velmurugan GV, Sundaresan NR, Gupta MP, White C. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc Res. 2013; 100:143–150.31. Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 2014; 63:605–618.32. Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013; 33:2996–3010.33. Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Huang H, Wu L, Eberhart C, Handa JT, Du Y, Kern TS, Thimmulappa R, Barber AJ, Biswal S, Duh EJ. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014; 57:204–213.34. Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013; 54:3941–3948.35. Miao X, Cui W, Sun W, Xin Y, Wang B, Tan Y, Cai L, Miao L, Fu Y, Su G, Wang Y. Therapeutic effect of MG132 on the aortic oxidative damage and inflammatory response in OVE26 type 1 diabetic mice. Oxid Med Cell Longev. 2013; 2013:879516.36. Wang Y, Sun W, Du B, Miao X, Bai Y, Xin Y, Tan Y, Cui W, Liu B, Cui T, Epstein PN, Fu Y, Cai L. Therapeutic effect of MG-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: roles of Nrf2 and NF-kappaB. Am J Physiol Heart Circ Physiol. 2013; 304:H567–H578.37. Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011; 60:3055–3066.38. Cui W, Bai Y, Miao X, Luo P, Chen Q, Tan Y, Rane MJ, Miao L, Cai L. Prevention of diabetic nephropathy by sulforaphane: possible role of nrf2 upregulation and activation. Oxid Med Cell Longev. 2012; 2012:821936.39. Miao X, Bai Y, Sun W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its downstream antioxidants. Nutr Metab (Lond). 2012; 9:84.40. Cui W, Li B, Bai Y, Miao X, Chen Q, Sun W, Tan Y, Luo P, Zhang C, Zheng S, Epstein PN, Miao L, Cai L. Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab. 2013; 304:E87–E99.41. Miao X, Wang Y, Sun J, Sun W, Tan Y, Cai L, Zheng Y, Su G, Liu Q, Wang Y. Zinc protects against diabetes-induced pathogenic changes in the aorta: roles of metallothionein and nuclear factor (erythroid-derived 2)-like 2. Cardiovasc Diabetol. 2013; 12:54.42. Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013; 57:82–95.43. Chin M, Lee CY, Chuang JC, Bumeister R, Wigley WC, Sonis ST, Ward KW, Meyer C. Bardoxolone methyl analogs RTA 405 and dh404 are well tolerated and exhibit efficacy in rodent models of type 2 diabetes and obesity. Am J Physiol Renal Physiol. 2013; 304:F1438–F1446.44. Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L, Sun J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev. 2014; 2014:123963.45. Negi G, Kumar A, Sharma SS. Nrf2 and NF-kappaB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res. 2011; 8:294–304.46. Luo ZF, Qi W, Feng B, Mu J, Zeng W, Guo YH, Pang Q, Ye ZL, Liu L, Yuan FH. Prevention of diabetic nephropathy in rats through enhanced renal antioxidative capacity by inhibition of the proteasome. Life Sci. 2011; 88:512–520.47. Shin S, Wakabayashi J, Yates MS, Wakabayashi N, Dolan PM, Aja S, Liby KT, Sporn MB, Yamamoto M, Kensler TW. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur J Pharmacol. 2009; 620:138–144.48. Tan SM, Sharma A, Stefanovic N, Yuen DY, Karagiannis TC, Meyer C, Ward KW, Cooper ME, de Haan JB. A derivative of Bardoxolone methyl, dh404, in an inverse dose-dependent manner, lessens diabetes-associated atherosclerosis and improves diabetic kidney disease. Diabetes. 2014; 63:3091–3103.49. Liu Y, Wang Y, Miao X, Zhou S, Tan Y, Liang G, Zheng Y, Liu Q, Sun J, Cai L. Inhibition of JNK by compound C66 prevents pathological changes of the aorta in STZ-induced diabetes. J Cell Mol Med. 2014; 18:1203–1212.50. Abo-Salem OM, Harisa GI, Ali TM, El-Sayed ES, Abou-Elnour FM. Curcumin ameliorates streptozotocin-induced heart injury in rats. J Biochem Mol Toxicol. 2014; 28:263–270.51. Wang Y, Zhou S, Sun W, McClung K, Pan Y, Liang Q, Tan Y, Zhao Y, Liu Q, Sun J, Cai L. Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. Am J Physiol Endocrinol Metab. 2014; 306:E1239–E1247.52. Castro CN, Barcala Tabarrozzi AE, Winnewisser J, Gimeno ML, Antunica Noguerol M, Liberman AC, Paz DA, Dewey RA, Perone MJ. Curcumin ameliorates autoimmune diabetes. Evidences in accelerated murine models of type 1 diabetes. Clin Exp Immunol. 2014; 177:149–160.53. Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014; 25:144–150.54. Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, Yang CX. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014; 560:81–85.55. Thomas J, Garg ML, Smith DW. Dietary resveratrol supplementation normalizes gene expression in the hippocampus of streptozotocin-induced diabetic C57Bl/6 mice. J Nutr Biochem. 2014; 25:313–318.56. Sadi G, Bozan D, Yildiz HB. Redox regulation of antioxidant enzymes: post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol Cell Biochem. 2014; 393:111–122.57. Guo R, Liu B, Wang K, Zhou S, Li W, Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab Vasc Dis Res. 2014; 11:92–102.58. Bresciani L, Calani L, Bocchi L, Delucchi F, Savi M, Ray S, Brighenti F, Stilli D, Del Rio D. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr Metab Cardiovasc Dis. 2014; 24:408–415.59. Bashmakov YK, Assaad-Khalil SH, Abou Seif M, Udumyan R, Megallaa M, Rohoma KH, Zeitoun M, Petyaev IM. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014; 2014:816307.60. Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li J, Long R, Zhou Y. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in steptozotocin-induced type 2 diabetic rats: role of NF-kappa B signaling. Eur J Pharmacol. 2013; 720:147–157.61. Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. BEAM Study Investigators. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011; 365:327–336.62. de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM. BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013; 369:2492–2503.63. Zhang DD. Bardoxolone brings Nrf2-based therapies to light. Antioxid Redox Signal. 2013; 19:517–518.64. Himmelfarb J, Tuttle KR. New therapies for diabetic kidney disease. N Engl J Med. 2013; 369:2549–2550.65. Li B, Liu S, Miao L, Cai L. Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res. 2012; 2012:216512.66. Ding Y, Stidham RD, Bumeister R, Trevino I, Winters A, Sprouse M, Ding M, Ferguson DA, Meyer CJ, Wigley WC, Ma R. The synthetic triterpenoid, RTA 405, increases the glomerular filtration rate and reduces angiotensin II-induced contraction of glomerular mesangial cells. Kidney Int. 2013; 83:845–854.67. Hsu WH, Lee BH, Chang YY, Hsu YW, Pan TM. A novel natural Nrf2 activator with PPARgamma-agonist (monascin) attenuates the toxicity of methylglyoxal and hyperglycemia. Toxicol Appl Pharmacol. 2013; 272:842–851.68. Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012; 61:3208–3218.69. Zhang YK, Wu KC, Liu J, Klaassen CD. Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicol Appl Pharmacol. 2012; 264:305–314.70. Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal. 2011; 14:957–971.71. Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovasc Res. 2013; 100:63–73.72. Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010; 106:463–478.73. Marchan R, Bolt HM. The cytoprotective and the dark side of Nrf2. Arch Toxicol. 2013; 87:2047–2050.74. Grossman R, Ram Z. The dark side of Nrf2. World Neurosurg. 2013; 80:284–286.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Echocardiographic Diagnosis of Diabetic Cardiomyopathy
- Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
- Two Cases of Hypertrophic Cardiomyopathy in the Infants of Diabetic Mother
- An autopssy case of infant of diabetic mother with d-transposition of great arteries and hypertrophic cardiomyopathy
- Diabetic Cardiomyopathy