Clin Exp Otorhinolaryngol.
2014 Mar;7(1):13-18.
Protective Effect of Hexane and Ethanol Extract of Piper Longum L. on Gentamicin-Induced Hair Cell Loss in Neonatal Cultures
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Dongguk University Ilsan Hospital, Goyang, Korea. jjsong23@gmail.com
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College of Medicine, Seoul, Korea.
Abstract
OBJECTIVES
Gentamicin (GM) is a commonly used aminoglycoside antibiotic that generates free oxygen radicals within the inner ear, which can cause vestibulo-cochlear toxicity and permanent damage to the sensory hair cells and neurons. Piper longum L. (PL) is a well-known spice and traditional medicine in Asia and Pacific islands, which has been reported to exhibit a wide spectrum of activity, including antioxidant activity. In this study, we evaluated the effect of hexane:ethanol (2:8) PL extract (subfraction of PL [SPL] extract) on GM-induced hair cell loss in basal, middle and apical regions in a neonatal cochlea cultures.
METHODS
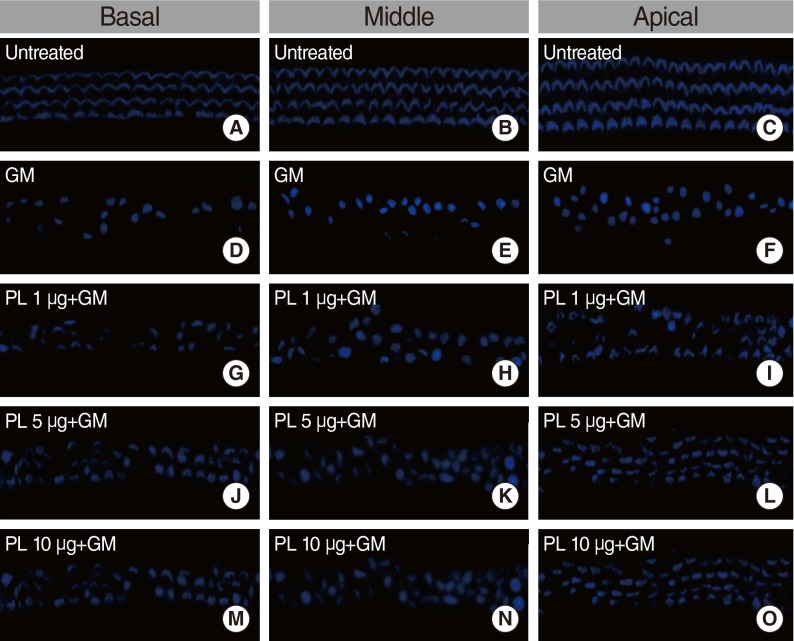
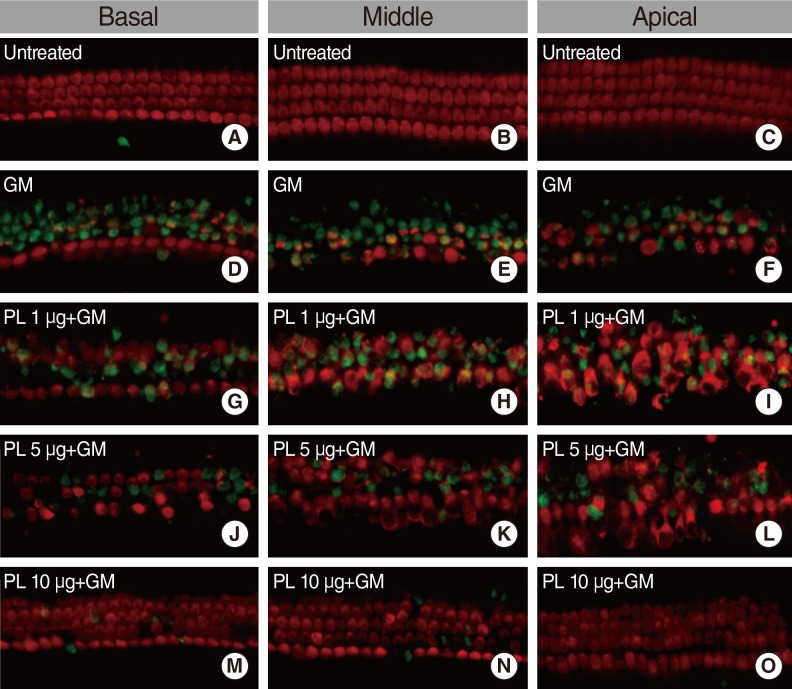
The protective effects of SPL extract were measured by phalloidin staining of cultures from postnatal day 2-3 mice with GM-induced hair cell loss. The anti-apoptosis activity of SPL extract was measured using double labeling by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and myosin-7a staining. The radical-scavenging activity of SPL extract was assessed using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay.
RESULTS
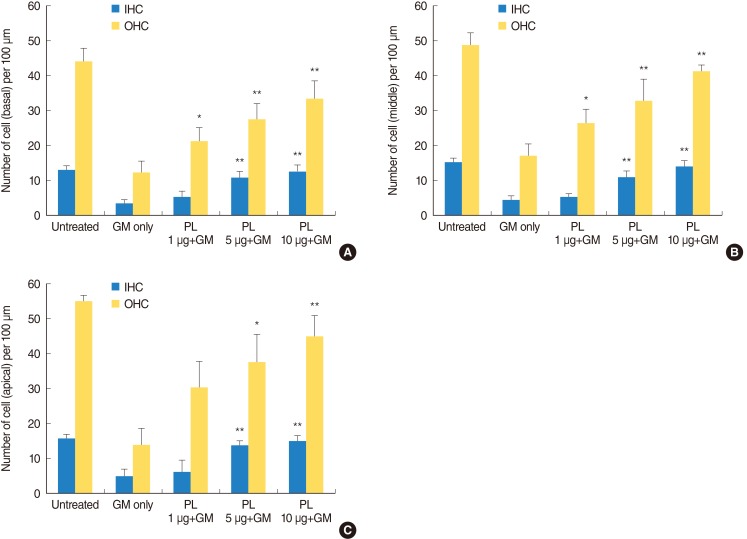
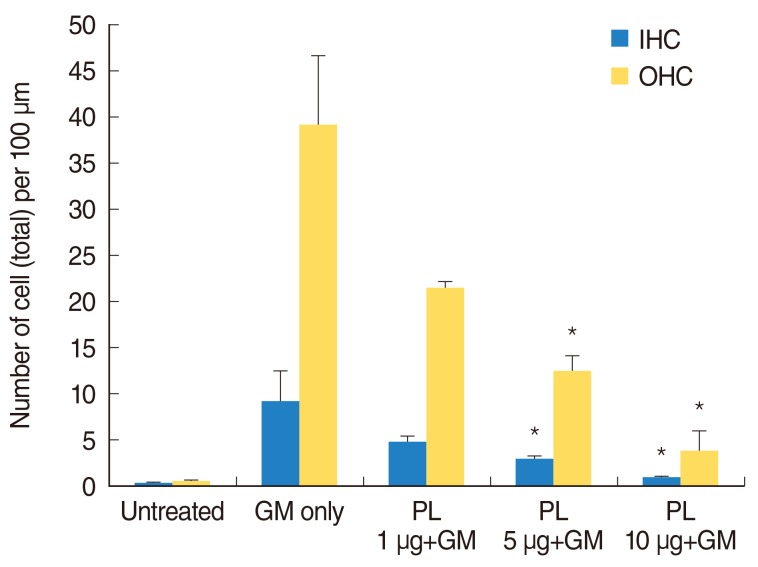
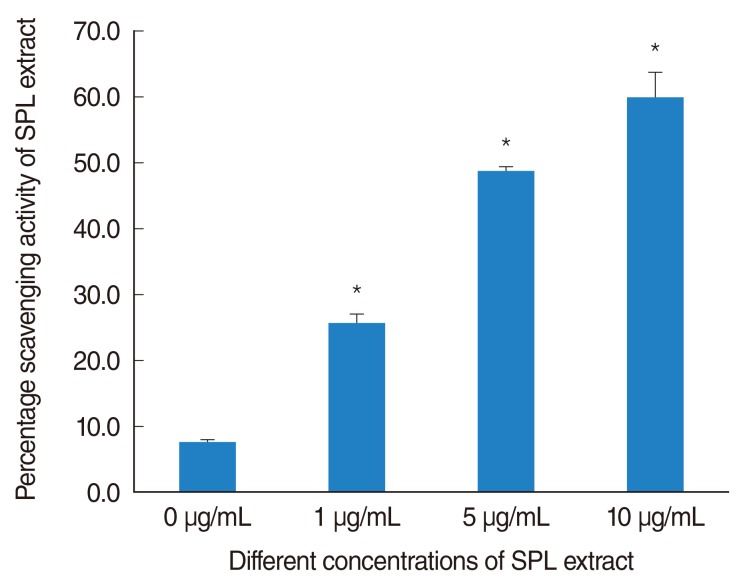
SPL extract at a concentration of 1 microg/mL significantly inhibited GM-induced hair cell loss at basal and middle region of cochlea, while 5 microg/mL was effective against apical region hair cell loss. The protective effect of SPL extract was concentration dependent and hair cells retained their stereocilia in explants treated with SPL extract prior to treatment with 0.3 mM GM. SPL extract decreased GM-induced apoptosis of hair cells as assessed by TUNEL staining. The outer hair and inner hair counts were not decreased in SPL extract treated groups in compare to GM treated explants. Additionally, SPL extract showed concentration dependent radical scavenging activity in a DPPH assay.
CONCLUSION
An anti-apoptosis effect and potent radical scavenger activity of SPL extract protects from GM-induced hair cell loss at basal, middle and apical regions in neonatal cochlea cultures.
Keyword
MeSH Terms
-
Animals
Apoptosis
Asia
Cochlea
DNA Nucleotidylexotransferase
Ear, Inner
Ethanol*
Gentamicins
Hair*
In Situ Nick-End Labeling
Medicine, Traditional
Mice
Neurons
Pacific Islands
Phalloidine
Piper*
Reactive Oxygen Species
Spices
Stereocilia
DNA Nucleotidylexotransferase
Ethanol
Gentamicins
Phalloidine
Reactive Oxygen Species
Figure
Reference
-
1. Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995; 11. 50(11):1749–1752. PMID: 8615852.
Article2. Kim JB, Jung JY, Ahn JC, Rhee CK, Hwang HJ. Antioxidant and anti-apoptotic effect of melatonin on the vestibular hair cells of rat utricles. Clin Exp Otorhinolaryngol. 2009; 3. 2(1):6–12. PMID: 19434285.
Article3. Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000; 1. 139(1-2):97–115. PMID: 10601716.
Article4. Fetoni AR, Sergi B, Scarano E, Paludetti G, Ferraresi A, Troiani D. Protective effects of alpha-tocopherol against gentamicin-induced Oto-vestibulo toxicity: an experimental study. Acta Otolaryngol. 2003; 1. 123(2):192–197. PMID: 12701739.5. Conlon BJ, Aran JM, Erre JP, Smith DW. Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res. 1999; 2. 128(1-2):40–44. PMID: 10082281.6. Takumida M, Popa R, Anniko M. Free radicals in the guinea pig inner ear following gentamicin exposure. ORL J Otorhinolaryngol Relat Spec. 1999; Mar-Apr. 61(2):63–70. PMID: 10095194.
Article7. Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae extract) preparations attenuate aminoglycoside-induced free radical formation in vitro and ototoxicity in vivo. Antimicrob Agents Chemother. 2003; 6. 47(6):1836–1841. PMID: 12760856.8. Jung HW, Chang SO, Kim CS, Rhee CS, Lim DH. Effects of Ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs. J Korean Med Sci. 1998; 10. 13(5):525–528. PMID: 9811183.
Article9. Long M, Smouha EE, Qiu D, Li F, Johnson F, Luft B. Flavanoid of Drynaria fortunei protects against gentamicin ototoxicity. Phytother Res. 2004; 8. 18(8):609–614. PMID: 15476311.10. Du XF, Song JJ, Hong S, Kim J. Ethanol extract of Piper longum L. attenuates gentamicin-induced hair cell loss in neonatal cochlea cultures. Pharmazie. 2012; 6. 67(6):559–563. PMID: 22822547.11. Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000; Jan-Feb. 5(1):3–22. PMID: 10686428.
Article12. Theopold HM. Comparative surface studies of ototoxic effects of various aminoglycoside antibiotics on the organ of Corti in the guinea pig: a scanning electron microscopic study. Acta Otolaryngol. 1977; Jul-Aug. 84(1-2):57–64. PMID: 899753.
Article13. Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Lab Invest. 1999; 7. 79(7):807–813. PMID: 10418821.14. Schacht J. Antioxidant therapy attenuates aminoglycoside-induced hearing loss. Ann N Y Acad Sci. 1999; 11. 884:125–130. PMID: 10842589.15. Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001; 5. 155(1-2):1–8. PMID: 11335071.
Article16. Choung YH, Kim SW, Tian C, Min JY, Lee HK, Park SN, et al. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope. 2011; 6. 121(6):1294–1302. PMID: 21541943.
Article17. Jeong HJ, Choi Y, Kim MH, Kang IC, Lee JH, Park C, et al. Rosmarinic acid, active component of Dansam-Eum attenuates ototoxicity of cochlear hair cells through blockage of caspase-1 activity. PLoS One. 2011; 4. 6(4):e18815. PMID: 21526214.
Article18. Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011; 12. 282(1-2):92–96. PMID: 21979311.19. Park MK, Lee BD, Chae SW, Chi J, Kwon SK, Song JJ. Protective effect of NecroX, a novel necroptosis inhibitor, on gentamicin-induced ototoxicity. Int J Pediatr Otorhinolaryngol. 2012; 9. 76(9):1265–1269. PMID: 22704672.
Article20. Song BB, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res. 1996; 5. 94(1-2):87–93. PMID: 8789814.
Article21. Song BB, Sha SH, Schacht J. Iron chelators protect from aminoglycoside-induced cochleo- and vestibulo-toxicity. Free Radic Biol Med. 1998; 7. 25(2):189–195. PMID: 9667495.
Article22. Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011; 11. 281(1-2):28–37. PMID: 21640178.
Article23. Kumar S, Kamboj J, Suman , Sharma S. Overview for various aspects of the health benefits of Piper longum linn: fruit. J Acupunct Meridian Stud. 2011; 6. 4(2):134–140. PMID: 21704957.
Article24. Shankaracharya NB, Rao LJ, Naik JP, Nagalakshmi S. Characterization of chemical constituents of Indian Long Pepper (Piper-longum L). J Food Sci Tech. 1997; 34(1):73–75.25. Pathak N, Khandelwal S. Modulation of cadmium induced alterations in murine thymocytes by piperine: oxidative stress, apoptosis, phenotyping and blastogenesis. Biochem Pharmacol. 2006; 8. 72(4):486–497. PMID: 16780805.
Article26. Pradeep CR, Kuttan G. Effect of piperine on the inhibition of nitric oxide (NO) and TNF-alpha production. Immunopharmacol Immunotoxicol. 2003; 8. 25(3):337–346. PMID: 19180797.27. Jagdale SC, Kuchekar BS, Chabukswar AR, Lokhande PD, Raut CG. Anti-oxidant activity of Piper longum Linn. Int J Biol Chem. 2009; 3(3):119–125.
Article28. Joy B, Sandhya CP, Remitha KR. Comparison and bioevaluation of Piper longum fruit extracts. J Chem Pharm Res. 2010; 2(4):696–706.29. Lokhande PD, Gawai KR, Kodam KM, Kuchekar BS, Chabukswar AR, Jagdale SC. Antibacterial activity of extracts of Piper longum. J Pharm Toxicol. 2007; 2(6):574–579.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Metformin on Gentamicin-Induced Vestibulotoxicity in Rat Primary Cell Culture
- Effects of Ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs
- Antioxidant and Anti-Apoptotic Effect of Melatonin on the Vestibular Hair Cells of Rat Utricles
- Effect of Hexane Extract of Galla Rhois on Inflammatory Alveolar Bone Loss
- Quantitative Analysis of Solvent Extracted Skin Surface Lipid in Human Skin ( - hexane, hexane/ methanol, ethanol - )