Korean J Pain.
2014 Jul;27(3):239-245. 10.3344/kjp.2014.27.3.239.
Intrathecal Administration of Mesenchymal Stem Cells Reduces the Reactive Oxygen Species and Pain Behavior in Neuropathic Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, School of Medicine, Chungnam National University, Daejeon, Korea. whlee@cnu.ac.kr
- 2Department of Microbiology, School of Medicine, Chungnam National University, Daejeon, Korea. songch@cnu.ac.kr
- KMID: 2278230
- DOI: http://doi.org/10.3344/kjp.2014.27.3.239
Abstract
- BACKGROUND
Neuropathic pain induced by spinal or peripheral nerve injury is very resistant to common pain killers, nerve block, and other pain management approaches. Recently, several studies using stem cells suggested a new way to control the neuropatic pain. In this study, we used the spinal nerve L5 ligation (SNL) model to investigate whether intrathecal rat mesenchymal stem cells (rMSCs) were able to decrease pain behavior, as well as the relationship between rMSCs and reactive oxygen species (ROS).
METHODS
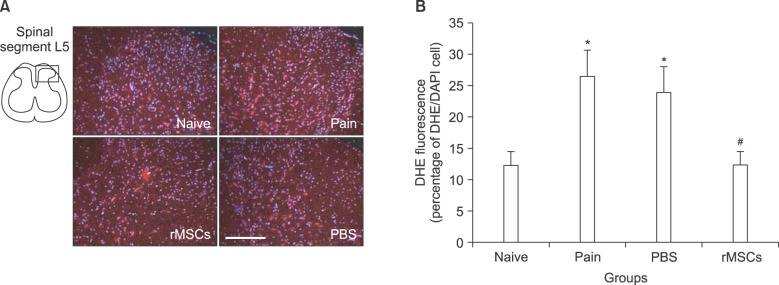
Neuropathic pain of the left hind paw was induced by unilateral SNL in Sprague-Dawley rats (n = 10 in each group). Mechanical sensitivity was assessed using Von Frey filaments at 3, 7, 10, 12, 14, 17, and 24 days post-ligation. rMSCs (10 microl, 1 x 105) or phosphate buffer saline (PBS, 10 microl) was injected intrathecally at 7 days post-ligation. Dihydroethidium (DHE), an oxidative fluorescent dye, was used to detect ROS at 24 days post-ligation.
RESULTS
Tight ligation of the L5 spinal nerve induced allodynia in the left hind paw after 3 days post-ligation. ROS expression was increased significantly (P < 0.05) in spinal dorsal horn of L5. Intrathecal rMSCs significantly (P < 0.01) alleviated the allodynia at 10 days after intrathecal injection (17 days post-ligation). Intrathecal rMSCs administration significantly (P < 0.05) reduced ROS expression in the spinal dorsal horn.
CONCLUSIONS
These results suggest that rMSCs may modulate neuropathic pain generation through ROS expression after spinal nerve ligation.
MeSH Terms
Figure
Cited by 3 articles
-
Stem cell therapy in pain medicine
Yong Hee Han, Kyung Hoon Kim, Salahadin Abdi, Tae Kyun Kim
Korean J Pain. 2019;32(4):245-255. doi: 10.3344/kjp.2019.32.4.245.Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats
Ahmed Olalekan Bakare, Bamidele Victor Owoyele
Korean J Pain. 2020;33(1):13-22. doi: 10.3344/kjp.2020.33.1.13.The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice
Sie Hyeon Yoo, Sung Hyun Lee, Seunghwan Lee, Jae Hong Park, Seunghyeon Lee, Heecheol Jin, Hue Jung Park
Korean J Pain. 2020;33(1):23-29. doi: 10.3344/kjp.2020.33.1.23.
Reference
-
1. Gilron I, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006; 175:265–275. PMID: 16880448.
Article2. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000; 275:9645–9652. PMID: 10734116.
Article3. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999; 181:67–73. PMID: 10457354.
Article4. Lu L, Zhao C, Liu Y, Sun X, Duan C, Ji M, et al. Therapeutic benefit of TH-engineered mesenchymal stem cells for Parkinson's disease. Brain Res Brain Res Protoc. 2005; 15:46–51. PMID: 15878150.
Article5. Yang LY, Huang TH, Ma L. Bone marrow stromal cells express neural phenotypes in vitro and migrate in brain after transplantation in vivo. Biomed Environ Sci. 2006; 19:329–335. PMID: 17190183.6. Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005; 7:36–45. PMID: 16040382.
Article7. Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004; 111:116–124. PMID: 15327815.
Article8. Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004; 309:869–878. PMID: 14988418.
Article9. Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010; 19:1885–1893. PMID: 20380515.
Article10. Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994; 57:375–382. PMID: 7936715.
Article11. Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009; 4:102–106. PMID: 19131962.
Article12. Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008; 3:e3336. PMID: 18852872.
Article13. Papir-Kricheli D, Frey J, Laufer R, Gilon C, Chorev M, Selinger Z, et al. Behavioural effects of receptor-specific substance P agonists. Pain. 1987; 31:263–276. PMID: 2448728.
Article14. Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008; 118:659–670. PMID: 18219391.
Article15. Kumar S, Ruchi R, James SR, Chidiac EJ. Gene therapy for chronic neuropathic pain: how does it work and where do we stand today? Pain Med. 2011; 12:808–822. PMID: 21564510.
Article16. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005; 6:521–532. PMID: 15995723.
Article17. Hill RG. Molecular basis for the perception of pain. Neuroscientist. 2001; 7:282–292. PMID: 11488394.18. Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007; 10:1361–1368. PMID: 17965656.
Article19. Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009; 32:611–618. PMID: 19781793.
Article20. Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A. 2010; 107:14851–14856. PMID: 20679217.
Article21. Siniscalco D, Giordano C, Rossi F, Maione S, de Novellis V. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011; 9:523–529. PMID: 22654713.
Article22. Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004; 116:639–648. PMID: 15006347.
Article23. Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010; 67:655–669. PMID: 19937263.
Article24. Savitz SI, Dinsmore JH, Wechsler LR, Rosenbaum DM, Caplan LR. Cell therapy for stroke. NeuroRx. 2004; 1:406–414. PMID: 15717044.
Article25. Levy YS, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y, et al. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson's disease. Cytotherapy. 2008; 10:340–352. PMID: 18574767.
Article26. Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004; 15:1105–1108. PMID: 15129154.
Article27. Kim CH, Kim YW, Jang SH, Chang CH, Jung JH, Kim SH. Motor function recovery after adipose tissue derived mesenchymal stem cell therapy in rats with cerebral infarction. J Korean Neurosurg Soc. 2006; 40:267–272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiallodynic effect of intrathecal epigallocatechin-3-gallate due to suppression of reactive oxygen species
- Antinociceptive Effects of Intraperitoneal and Intrathecal Vitamin E in the Rat Formalin Test
- Antinociceptive effects of vitamin E in formalin-induced nociceptive response in rats
- Antinociceptive effect of phenyl N-tert-butylnitrone, a free radical scavenger, on the rat formalin test
- Reactive Oxygen Species and Cancer