Korean J Pain.
2014 Jan;27(1):23-29. 10.3344/kjp.2014.27.1.23.
Spinal Noradrenergic Modulation and the Role of the Alpha-2 Receptor in the Antinociceptive Effect of Intrathecal Nefopam in the Formalin Test
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Medical School and Hospital, Gwangju, Korea. jichoi@jnu.ac.kr
- KMID: 2278198
- DOI: http://doi.org/10.3344/kjp.2014.27.1.23
Abstract
- BACKGROUND
Nefopam has shown an analgesic effect on acute pain including postoperative pain. The reuptake of monoamines including serotonin and noradrenaline has been proposed as the mechanism of the analgesic action of nefopam, but it remains unclear. Although alpha-adrenergic agents are being widely used in the perioperative period, the role of noradrenergic modulation in the analgesic effect of nefopam has not been fully addressed.
METHODS
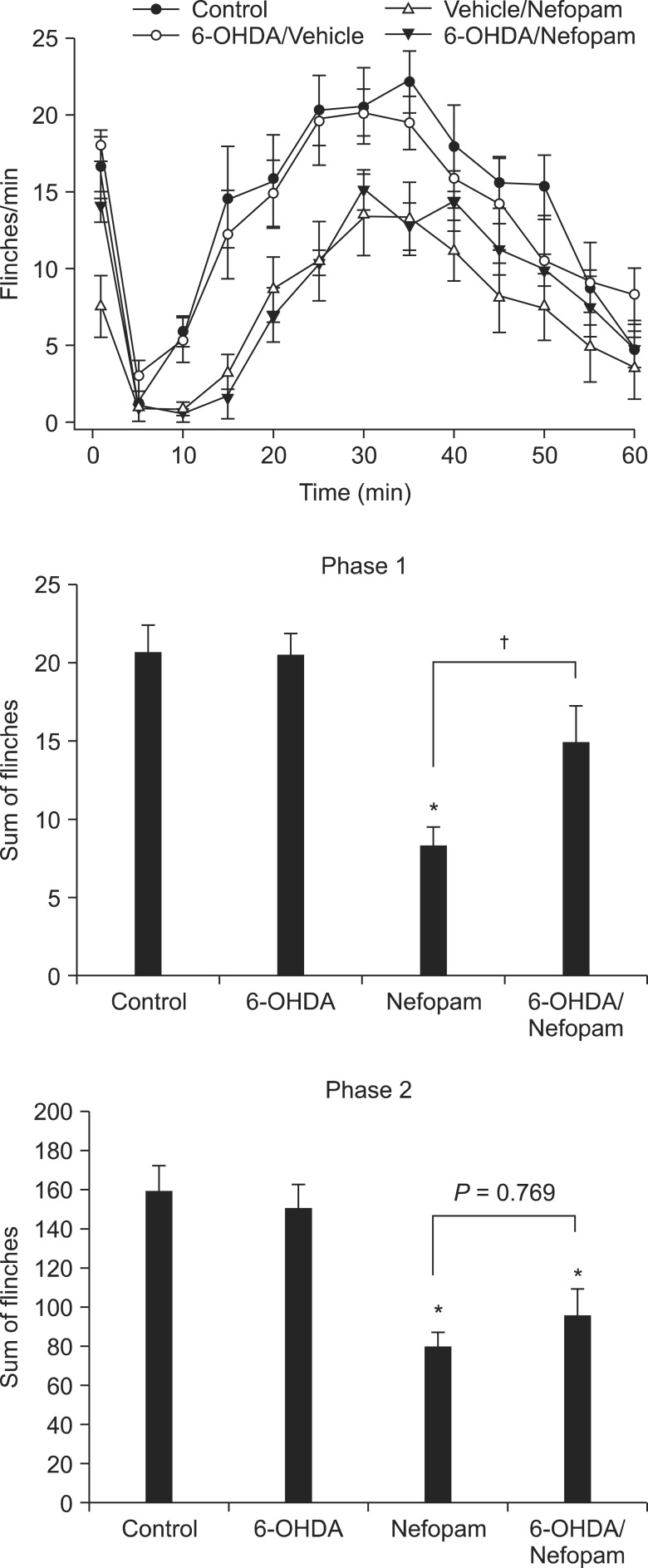
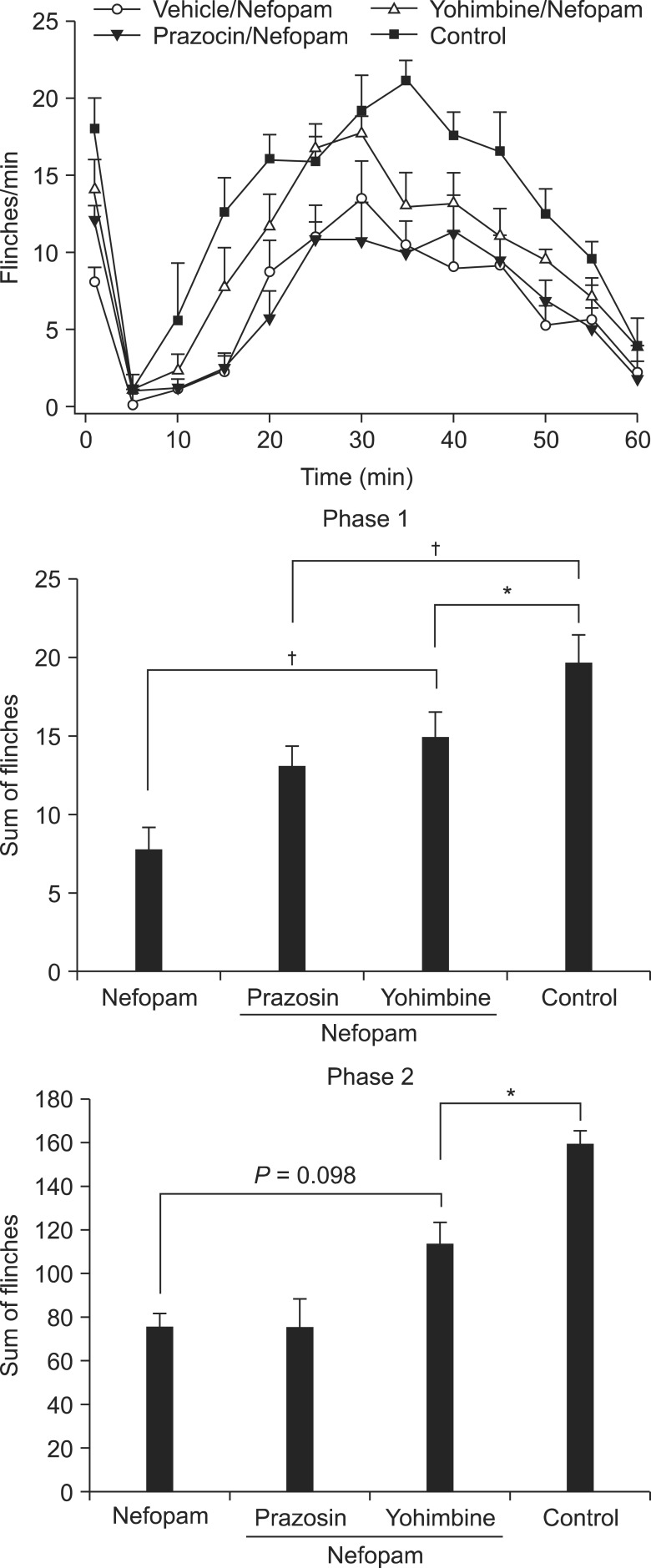
Changes in the antinociceptive effect of intrathecal (i.t.) nefopam against formalin-elicited flinching responses were explored in Sprague-Dawley rats pretreated with i.t. 6-hydroxydopamine (6-OHDA), which depletes spinal noradrenaline. In addition, antagonism to the effect of nefopam by prazosin and yohimbine was evaluated to further elucidate the antinociceptive mechanism of i.t. nefopam.
RESULTS
Pretreatment with i.t. 6-OHDA alone did not alter the flinching responses in either phase of the formalin test, while it attenuated the antinociceptive effect of i.t. nefopam significantly during phase 1, but not phase 2. The antagonist of the alpha-2 receptor, but not the alpha-1 receptor, reduced partially, but significantly, the antinociceptive effect of i.t. nefopam during phase 1, but not during phase 2.
CONCLUSIONS
This study demonstrates that spinal noradrenergic modulation plays an important role in the antinociceptive effect of i.t. nefopam against formalin-elicited acute initial pain, but not facilitated pain, and this action involves the spinal alpha-2 but not the alpha-1 receptor.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Expression of the spinal 5-HT7 receptor and p-ERK pathway in the carrageenan inflammatory pain of rats
Soo Young Cho, Hyoung Gon Ki, Joung Min Kim, Jin Myung Oh, Ji Hoon Yang, Woong Mo Kim, Hyung Gon Lee, Myung Ha Yoon, Jeong Il Choi
Korean J Anesthesiol. 2015;68(2):170-174. doi: 10.4097/kjae.2015.68.2.170.The Role of Spinal Dopaminergic Transmission in the Analgesic Effect of Nefopam on Rat Inflammatory Pain
Do Yun Kim, Joo Wung Chae, Chang Hun Lim, Bong Ha Heo, Keun Suk Park, Hyung Gon Lee, Jeong Il Choi, Myung Ha Yoon, Woong Mo Kim
Korean J Pain. 2016;29(3):164-171. doi: 10.3344/kjp.2016.29.3.164.
Reference
-
1. Fuller RW, Snoddy HD. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology. 1993; 32:995–999. PMID: 7507578.
Article2. Rosland JH, Hole K. The effect of nefopam and its enantiomers on the uptake of 5-hydroxytryptamine, noradrenaline and dopamine in crude rat brain synaptosomal preparations. J Pharm Pharmacol. 1990; 42:437–438. PMID: 1979627.
Article3. Vonvoigtlander PF, Lewis RA, Neff GL, Triezenberg HJ. Involvement of biogenic amines with the mechanisms of novel analgesics. Prog Neuropsychopharmacol Biol Psychiatry. 1983; 7:651–656. PMID: 6141608.
Article4. Esposito E, Romandini S, Merlo-Pich E, Mennini T, Samanin R. Evidence of the involvement of dopamine in the analgesic effect of nefopam. Eur J Pharmacol. 1986; 128:157–164. PMID: 3098570.
Article5. Hunskaar S, Fasmer OB, Broch OJ, Hole K. Involvement of central serotonergic pathways in nefopam-induced antinociception. Eur J Pharmacol. 1987; 138:77–82. PMID: 2442003.
Article6. Girard P, Coppé MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006; 54:195–202. PMID: 16750379.
Article8. Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. 2008; 71:217–221. PMID: 18625968.
Article9. Cho SY, Park AR, Yoon MH, Lee HG, Kim WM, Choi JI. Antinociceptive effect of intrathecal nefopam and interaction with morphine in formalin-induced pain of rats. Korean J Pain. 2013; 26:14–20. PMID: 23342202.
Article10. Bernatzky G, Jurna I. Intrathecal injection of codeine, buprenorphine, tilidine, tramadol and nefopam depresses the tail-flick response in rats. Eur J Pharmacol. 1986; 120:75–80. PMID: 3753938.
Article11. Fasmer OB, Berge OG, Jørgensen HA, Hole K. Antinociceptive effects of (+/-)-, (+)- and (-)-nefopam in mice. J Pharm Pharmacol. 1987; 39:508–511. PMID: 2886617.
Article12. Girard P, Pansart Y, Gillardin JM. Nefopam potentiates morphine antinociception in allodynia and hyperalgesia in the rat. Pharmacol Biochem Behav. 2004; 77:695–703. PMID: 15099914.
Article13. Tramoni G, Viale JP, Cazals C, Bhageerutty K. Morphine-sparing effect of nefopam by continuous intravenous injection after abdominal surgery by laparotomy. Eur J Anaesthesiol. 2003; 20:990–992. PMID: 14690106.
Article14. Kranke P, Eberhart LH, Roewer N, Tramèr MR. Single-dose parenteral pharmacological interventions for the prevention of postoperative shivering: a quantitative systematic review of randomized controlled trials. Anesth Analg. 2004; 99:718–727. PMID: 15333401.
Article15. Lu KZ, Shen H, Chen Y, Li MG, Tian GP, Chen J. Ondansetron does not attenuate the analgesic efficacy of nefopam. Int J Med Sci. 201; 10:1790–1794. PMID: 24273453.
Article16. Höcker J, Gruenewald M, Meybohm P, Schaper C, Scholz J, Steinfath M, et al. Nefopam but not physostigmine affects the thermoregulatory response in mice via alpha(2)-adrenoceptors. Neuropharmacology. 2010; 58:495–500. PMID: 19744502.
Article17. Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992; 12:3665–3670. PMID: 1326610.
Article18. Suzuki T, Li YH, Mashimo T. The antiallodynic and antihyperalgesic effects of neurotropin in mice with spinal nerve ligation. Anesth Analg. 2005; 101:793–799. PMID: 16115993.
Article19. Uutela P, Reinilä R, Harju K, Piepponen P, Ketola RA, Kostiainen R. Analysis of intact glucuronides and sulfates of serotonin, dopamine, and their phase I metabolites in rat brain microdialysates by liquid chromatography-tandem mass spectrometry. Anal Chem. 2009; 81:8417–8425. PMID: 19772284.
Article20. Shin DJ, Jeong CW, Lee SH, Yoon MH. Receptors involved in the antinociception of intrathecal melatonin in formalin test of rats. Neurosci Lett. 2011; 494:207–210. PMID: 21396983.
Article21. Gutierrez T, Nackley AG, Neely MH, Freeman KG, Edwards GL, Hohmann AG. Effects of neurotoxic destruction of descending noradrenergic pathways on cannabinoid antinociception in models of acute and tonic nociception. Brain Res. 2003; 987:176–185. PMID: 14499961.
Article22. Shin DJ, Jeong CW, Lee SH, Yoon MH. Receptors involved in the antinociception of intrathecal melatonin in formalin test of rats. Neurosci Lett. 2011; 494:207–210. PMID: 21396983.
Article23. Marazziti D, Rotondo A, Ambrogi F, Cassano GB. Analgesia by nefopam: does it act through serotonin? Drugs Exp Clin Res. 1991; 17:259–261. PMID: 1756689.24. Ohkubo Y, Nomura K, Yamaguchi I. Involvement of dopamine in the mechanism of action of FR64822, a novel non-opioid antinociceptive compound. Eur J Pharmacol. 1991; 204:121–125. PMID: 1839620.
Article25. Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004; 1013:249–255. PMID: 15193535.
Article26. Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005; 28:183–191. PMID: 15714253.
Article27. Verleye M, Gillardin JM. Contribution of transient receptor potential vanilloid subtype 1 to the analgesic and antihyperalgesic activity of nefopam in rodents. Pharmacology. 2009; 83:116–121. PMID: 19096234.
Article28. Nakajima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain. 2012; 153:990–997. PMID: 22424692.
Article29. Romero TR, Resende LC, Guzzo LS, Duarte ID. CB1 and CB2 cannabinoid receptor agonists induce peripheral antinociception by activation of the endogenous noradrenergic system. Anesth Analg. 2013; 116:463–472. PMID: 23302980.
Article30. Wada T, Hasegawa Y, Ono H. Characterization of alpha1-adrenoceptor subtypes in facilitation of rat spinal motoneuron activity. Eur J Pharmacol. 1997; 340:45–52. PMID: 9527505.
Article31. Yaksh TL. Central pharmacology of nociceptive transmission. In : McMahon SB, Koltzenburg M, editors. Wall and Melzack's textbook of pain. 5th ed. Philadelphia (PA): Elsevier;2006. p. 371–414.32. Day HE, Campeau S, Watson SJ Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997; 13:115–139. PMID: 9285356.
Article33. Gil DW, Cheevers CV, Kedzie KM, Manlapaz CA, Rao S, Tang E, et al. Alpha-1-adrenergic receptor agonist activity of clinical alpha-adrenergic receptor agonists interferes with alpha-2-mediated analgesia. Anesthesiology. 2009; 110:401–407. PMID: 19194166.
Article34. Van Elstraete AC, Sitbon P. Median effective dose (ED50) of paracetamol and nefopam for postoperative pain: isobolographic analysis of their antinociceptive interaction. Minerva Anestesiol. 2013; 79:232–239. PMID: 23241734.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
- Effect of Serotonergic Receptors on the Antinociception of Intrathecal Gabapentin in the Formalin Test of Rats
- The Role of Spinal Dopaminergic Transmission in the Analgesic Effect of Nefopam on Rat Inflammatory Pain
- The Role of Adrenergic and Cholinergic Receptors on the Antinociception of Intrathecal Zaprinast in the Formalin Test of Rats
- Antinociceptive Effects of Intrathecal Adenosine Receptors Subtype Agonists in the Formalin Test