Ann Dermatol.
2014 Feb;26(1):79-87. 10.5021/ad.2014.26.1.79.
Expression of Sfrp2 Is Increased in Catagen of Hair Follicles and Inhibits Keratinocyte Proliferation
- Affiliations
-
- 1Department of Medical Lifesciences, The Catholic University of Korea, School of Medicine, Seoul, Korea. sjkyoon@catholic.ac.kr
- KMID: 2265701
- DOI: http://doi.org/10.5021/ad.2014.26.1.79
Abstract
- BACKGROUND
Hair follicles undergo cycles of repeated growth and regression. The Wnt pathway plays an important role in the regeneration and differentiation of hair follicles. Sfrp2, a Wnt inhibitor, is involved in the developmental and disease processes of various cells and tissues by modulating the Wnt pathway.
OBJECTIVE
The aim of this study was to understand the role of Sfrp2 in hair follicles through investigation of the Sfrp2 expression pattern in the skin and its effect on keratinocytes.
METHODS
We investigated Sfrp2 mRNA expression and the expression of the wnt target genes, Ccnd1 and C-myc, at various mouse hair follicle developmental stages using Real-time polymerase chain reaction. We also investigated the effect of SFRP2 on the proliferation and differentiation of mouse keratinocyte cells by adding SFRP2 protein or overexpressing Sfrp2 using an in vitro culture system.
RESULTS
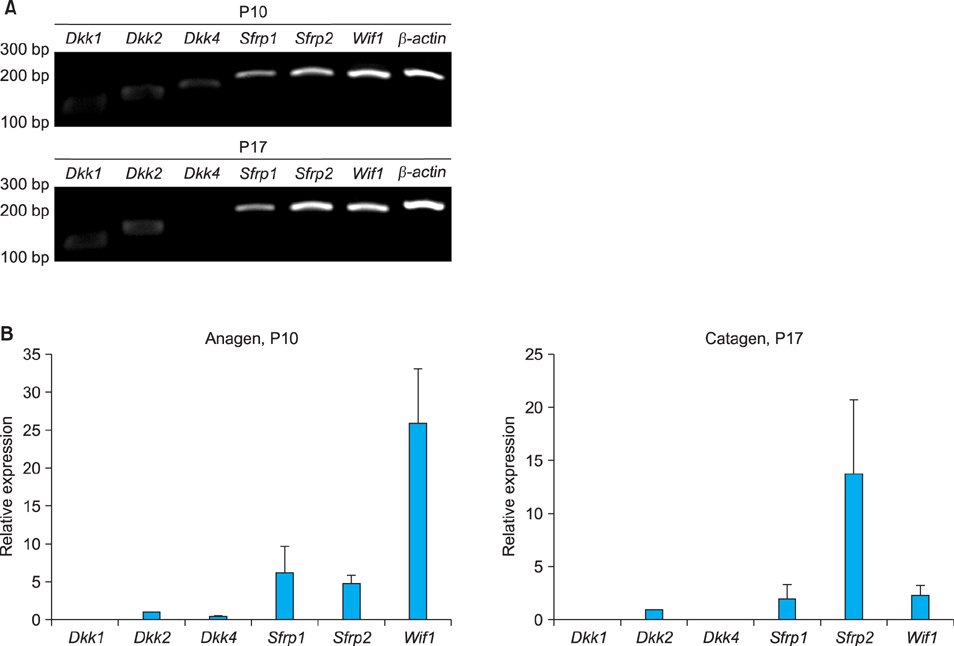
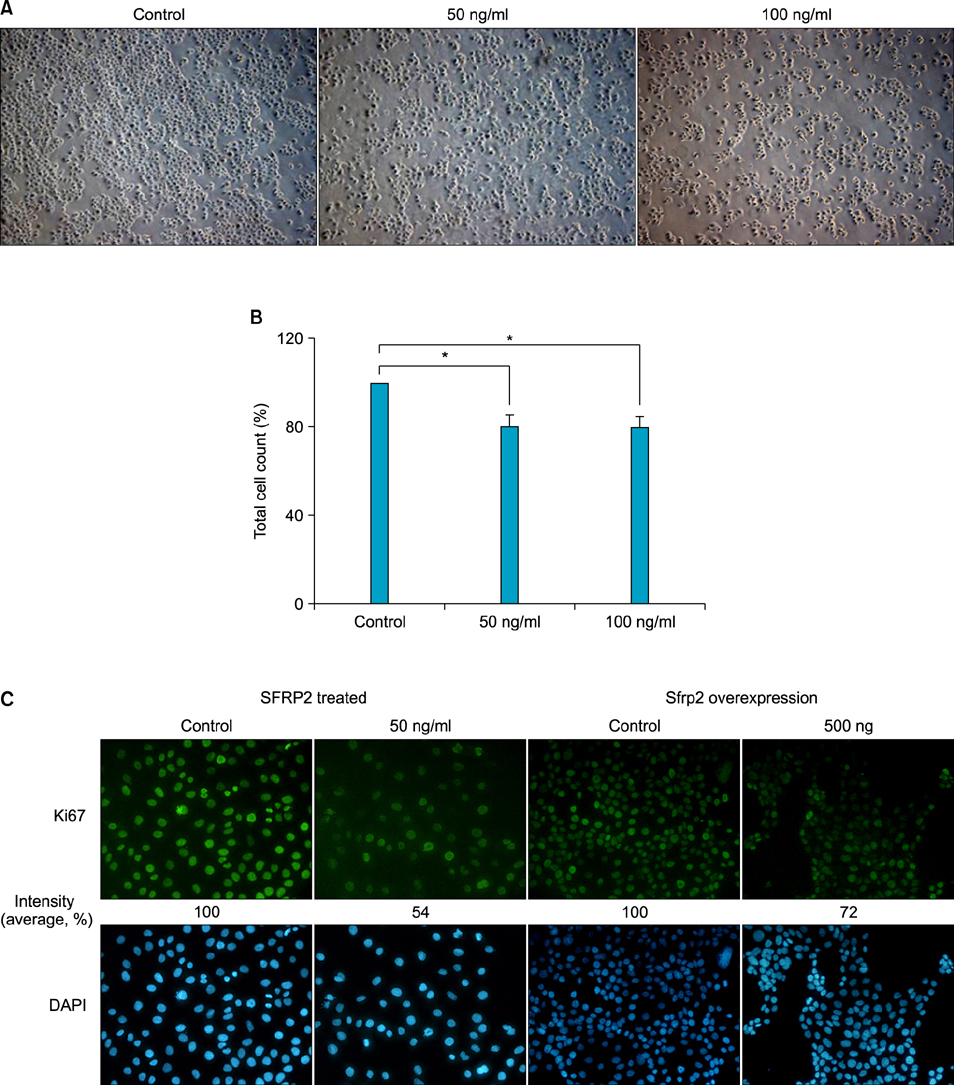
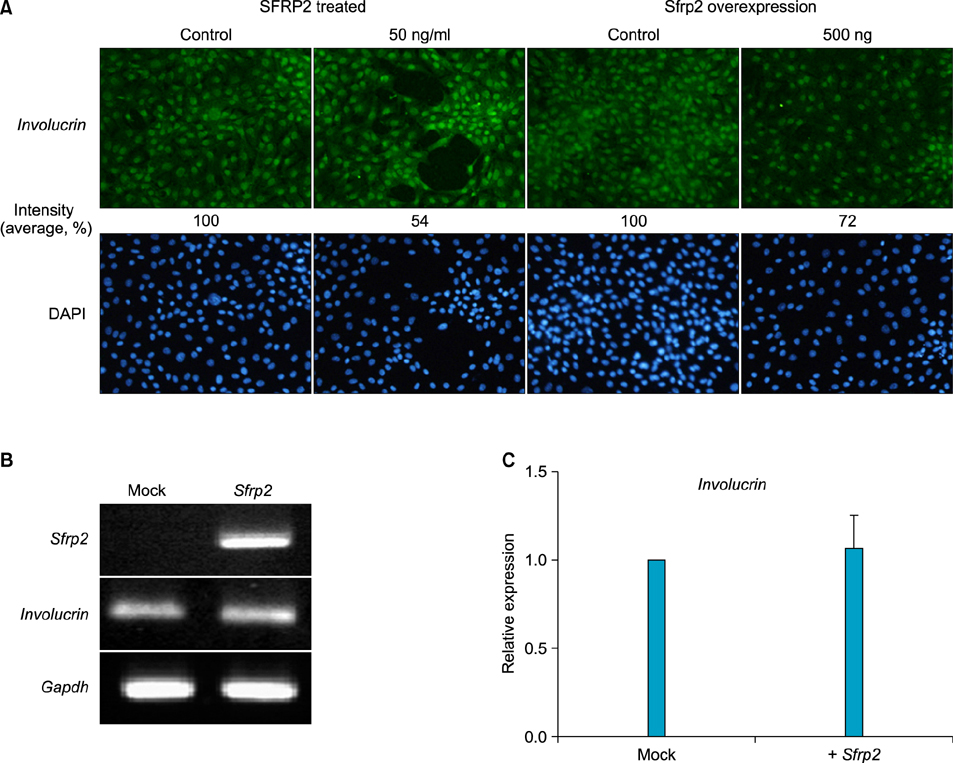
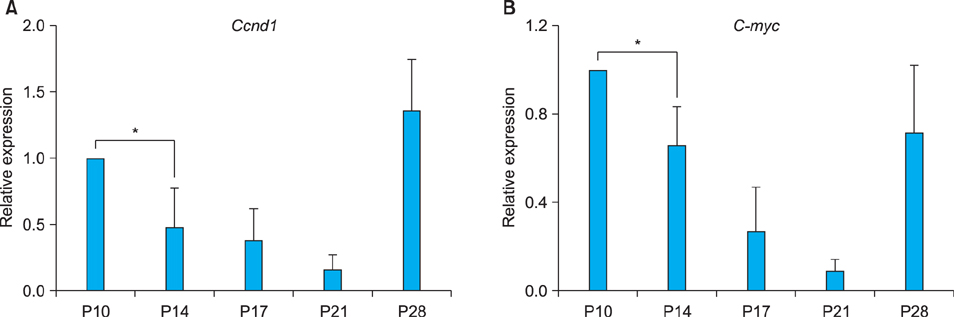
Sfrp2 expression peaked in the catagen phase and remained high until telogen, and then declined at the beginning of the next anagen. An inverse relationship to Sfrp2 expression was found for the expression of the Wnt target genes, C-myc and Ccnd1. In addition, we also observed inhibited proliferation of mouse keratinocytes in the presence of SFRP2.
CONCLUSION
These results suggest that Sfrp2 may play a role in the catagen phase by inhibiting the proliferation of keratinocyte and functioning as a Wnt inhibitor in keratinocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001; 81:449–494.
Article2. Callahan CA, Oro AE. Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling. Curr Opin Genet Dev. 2001; 11:541–546.
Article3. Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003; 17:1189–1200.
Article4. Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004; 72:512–526.
Article5. Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech Dev. 2004; 121:157–171.
Article6. Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002; 2:643–653.
Article7. Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004; 303:1483–1487.8. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004; 20:781–810.
Article9. Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006; 314:1447–1450.
Article10. Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005; 27:247–261.
Article11. Paus R, Foitzik K. In search of the "hair cycle clock": a guided tour. Differentiation. 2004; 72:489–511.
Article12. Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003; 116:2627–2634.
Article13. Ezan J, Leroux L, Barandon L, Dufourcq P, Jaspard B, Moreau C, et al. FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res. 2004; 63:731–738.
Article14. Hoang B, Moos M Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem. 1996; 271:26131–26137.
Article15. Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996; 382:225–230.16. Deb A, Davis BH, Guo J, Ni A, Huang J, Zhang Z, et al. SFRP2 regulates cardiomyogenic differentiation by inhibiting a positive transcriptional autofeedback loop of Wnt3a. Stem Cells. 2008; 26:35–44.17. Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A. 2008; 105:18366–18371.18. Descamps S, Arzouk H, Bacou F, Bernardi H, Fedon Y, Gay S, et al. Inhibition of myoblast differentiation by Sfrp1 and Sfrp2. Cell Tissue Res. 2008; 332:299–306.19. Rutberg SE, Kolpak ML, Gourley JA, Tan G, Henry JP, Shander D. Differences in expression of specific biomarkers distinguish human beard from scalp dermal papilla cells. J Invest Dermatol. 2006; 126:2583–2595.20. Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005; 3:e331.21. Baek IC, Kim JK, Cho KH, Cha DS, Cho JW, Park JK, et al. A novel mutation in Hr causes abnormal hair follicle morphogenesis in hairpoor mouse, an animal model for Marie Unna Hereditary Hypotrichosis. Mamm Genome. 2009; 20:350–358.22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.23. Magerl M, Tobin DJ, Müller-Röver S, Hagen E, Lindner G, McKay IA, et al. Patterns of proliferation and apoptosis during murine hair follicle morphogenesis. J Invest Dermatol. 2001; 116:947–955.24. Maganga R, Giles N, Adcroft K, Unni A, Keeney D, Wood F, et al. Secreted Frizzled related protein-4 (sFRP4) promotes epidermal differentiation and apoptosis. Biochem Biophys Res Commun. 2008; 377:606–611.25. Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem. 2009; 284:1234–1241.26. Lee JL, Chang CJ, Wu SY, Sargan DR, Lin CT. Secreted frizzled-related protein 2 (SFRP2) is highly expressed in canine mammary gland tumors but not in normal mammary glands. Breast Cancer Res Treat. 2004; 84:139–149.27. Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, et al. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000; 218:183–198.28. von Marschall Z, Fisher LW. Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem Biophys Res Commun. 2010; 400:299–304.29. Krause K, Foitzik K. Biology of the hair follicle: the basics. Semin Cutan Med Surg. 2006; 25:2–10.
Article30. Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007; 447:316–320.
Article31. Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, et al. WNT signaling in the control of hair growth and structure. Dev Biol. 1999; 207:133–149.
Article32. Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998; 95:605–614.
Article33. Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008; 135:2161–2172.34. Kim JK, Kim E, Baek IC, Kim BK, Cho AR, Kim TY, et al. Overexpression of Hr links excessive induction of Wnt signaling to Marie Unna hereditary hypotrichosis. Hum Mol Genet. 2010; 19:445–453.
Article35. Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006; 133:989–999.
Article36. Cho SW, Her SJ, Sun HJ, Choi OK, Yang JY, Kim SW, et al. Differential effects of secreted frizzled-related proteins (sFRPs) on osteoblastic differentiation of mouse mesenchymal cells and apoptosis of osteoblasts. Biochem Biophys Res Commun. 2008; 367:399–405.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hair Cycle-associated Changes of the Immunological Markers in Human Hair Follicles
- Expression of p63 and its association with cell proliferation at different stages of murine hair follicle cycle
- The Analysis of the Expression of TGF-beta in Human Hair Follicles in vivo
- Expression Pattern and Role of Klotho in Human Hair Follicles
- Observation of Follicular Morphology of Alopecia Areata by the Duration of the Lesion