Allergy Asthma Immunol Res.
2014 May;6(3):234-241. 10.4168/aair.2014.6.3.234.
Highly Protective Association of MMP-2-1306C/T Promoter Polymorphism With Asthma in a North Indian Population: A Pilot Study
- Affiliations
-
- 1Department of Biotechnology, Panjab University, Chandigarh, India. jagtar72@gmail.com
- 2Department of Pulmonary Medicine, PGIMER, Chandigarh, India.
- KMID: 2260686
- DOI: http://doi.org/10.4168/aair.2014.6.3.234
Abstract
- PURPOSE
Asthma is the most prevalent disease in India according to the national survey conducted by NFHS 2 in 1998-1999. Matrix metalloproteinase-2 (MMP-2), a collagenase encoded by the MMP-2 gene, degrades the type IV collagen and is responsible for inflammatory responses. This is a pilot study evaluating the role of MMP-2 -1306C/T promoter single nucleotide polymorphism (SNP) in asthma pathogenesis.
METHODS
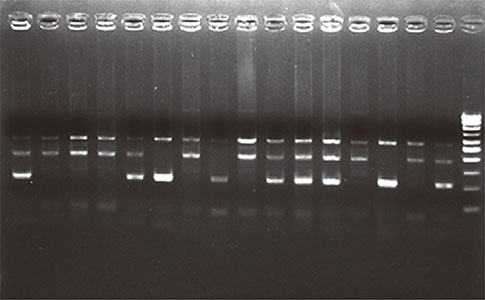
A case-control study was performed with a total of 824 adult subjects, including 410 adult asthmatics and 414 healthy controls from regions of North India. The MMP-2 -1306C/T polymorphism was genotyped by the Tetra-Primer Amplification Refractory Mutation System Polymerase Chain Reaction (Tetra-Primer ARMS PCR).
RESULTS
Statistical analysis of the results for the MMP-2 -1306C/T polymorphism revealed an extremely protective role of the mutant T allele in asthma pathogenesis with OR=0.45, 95% CI (0.35-0.58) and P=0.000. The heterozygous CT genotype also conferred protection from asthma with OR=0.37, 95% CI (0.27-0.51) and P=0.000. The homozygous TT genotype was also significantly associated with asthma with OR=0.35, 95% CI (0.16-0.72) and P=0.002. Moreover, the polymorphism was significantly associated with all the phenotypic traits of the disease.
CONCLUSION
The MMP-2 -1306C/T promoter polymorphism confers significant protection from asthma in the studied North Indian population
MeSH Terms
Figure
Reference
-
1. Holgate ST. Genetic and environmental interaction in allergy and asthma. J Allergy Clin Immunol. 1999; 104:1139–1146.2. Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999; 402:B5–B11.3. Sengler C, Lau S, Wahn U, Nickel R. Interactions between genes and environmental factors in asthma and atopy: new developments. Respir Res. 2002; 3:7.4. Elston RC. The genetic dissection of multifactorial traits. Clin Exp Allergy. 1995; 25:Suppl 2. 103–106.5. Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003; 167:78–82.6. Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003; 167:1360–1368.7. Jeffery PK, Laitinen A, Venge P. Biopsy markers of airway inflammation and remodelling. Respir Med. 2000; 94:Suppl F. S9–S15.8. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998; 339:1194–1200.9. Borger P, Tamm M, Black JL, Roth M. Asthma: is it due to an abnormal airway smooth muscle cell? Am J Respir Crit Care Med. 2006; 174:367–372.10. Knox AJ, Pang L, Johnson S, Hamad A. Airway smooth muscle function in asthma. Clin Exp Allergy. 2000; 30:606–614.11. Johnson SR, Knox AJ. Synthetic functions of airway smooth muscle in asthma. Trends Pharmacol Sci. 1997; 18:288–292.12. Johnson S, Knox A. Autocrine production of matrix metalloproteinase-2 is required for human airway smooth muscle proliferation. Am J Physiol. 1999; 277:L1109–L1117.13. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997; 378:151–160.14. National Library of Medicine (US). Entrez gene: MMP2 matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) [Internet]. Bethesda (MD): National Library of Medicine;2013. cited 2013 Nov 30. Available from: http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4313.15. Devarajan P, Johnston JJ, Ginsberg SS, Van Wart HE, Berliner N. Structure and expression of neutrophil gelatinase cDNA. Identity with type IV collagenase from HT1080 cells. J Biol Chem. 1992; 267:25228–25232.16. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004; 16:558–564.17. Cataldo D, Munaut C, Noël A, Frankenne F, Bartsch P, Foidart JM, Louis R. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000; 123:259–267.18. Global Initiative for Asthma (US). Global Initiative for Asthma (GINA) guidelines [Internet]. [place unknown]: Global Initiative for Asthma;2013. cited 2013 Nov 30. Available from: www.ginasthma.org.19. Guidelines for the measurement of respiratory function. Recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med. 1994; 88:165–194.20. National Heart, Lung, and Blood Institute (US). Calculate your body mass index [Internet]. Bethesda (MD): National Heart, Lung, and Blood Institute;2013. cited 2013 Nov 30. Available from: www.nhlbisupport.com/bmi/bmi-m.htm.21. Roe BA, Crabtree JS, Khan AS. Protocols for recombinant DNA isolation, cloning, and sequencing. In : Roe BA, editor. Methods for DNA isolation. Hoboken (NJ): John Wiley & Sons;1996. Available from: www.genome.ou.edu/protocol_book/protocol_partIII.html.22. Meijer MJ, Mieremet-Ooms MA, van Duijn W, van der Zon AM, Hanemaaijer R, Verheijen JH, van Hogezand RA, Lamers CB, Verspaget HW. Effect of the anti-tumor necrosis factor-alpha antibody infliximab on the ex vivo mucosal matrix metalloproteinase-proteolytic phenotype in inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:200–210.23. Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001; 29:e88.24. Xu J, Turner A, Little J, Bleecker ER, Meyers DA. Positive results in association studies are associated with departure from Hardy-Weinberg equilibrium: hint for genotyping error? Hum Genet. 2002; 111:573–574.25. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000; 1477:267–283.26. Kumagai K, Ohno I, Okada S, Ohkawara Y, Suzuki K, Shinya T, Nagase H, Iwata K, Shirato K. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999; 162:4212–4219.27. Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, Kobayashi T, Hataji O, Urano H, Zhou H, Suzuki K, Adachi Y. Thrombin in the airways of asthmatic patients. Lung. 1999; 177:253–262.28. Terada M, Kelly EA, Jarjour NN. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med. 2004; 169:373–377.29. Maisi P, Prikk K, Sepper R, Pirilä E, Salo T, Hietanen J, Sorsa T. Soluble membrane-type 1 matrix metalloproteinase (MT1-MMP) and gelatinase A (MMP-2) in induced sputum and bronchoalveolar lavage fluid of human bronchial asthma and bronchiectasis. APMIS. 2002; 110:771–782.30. Zervos EE, Shafii AE, Rosemurgy AS. Matrix metalloproteinase (MMP) inhibition selectively decreases type II MMP activity in a murine model of pancreatic cancer. J Surg Res. 1999; 81:65–68.31. Yang X, Staren ED, Howard JM, Iwamura T, Bartsch JE, Appert HE. Invasiveness and MMP expression in pancreatic carcinoma. J Surg Res. 2001; 98:33–39.32. Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer. 2001; 93:204–211.33. Gress TM, Müller-Pillasch F, Lerch MM, Friess H, Büchler M, Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995; 62:407–413.34. Koshiba T, Hosotani R, Wada M, Miyamoto Y, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998; 82:642–650.35. Bramhall SR, Stamp GW, Dunn J, Lemoine NR, Neoptolemos JP. Expression of collagenase (MMP2), stromelysin (MMP3) and tissue inhibitor of the metalloproteinases (TIMP1) in pancreatic and ampullary disease. Br J Cancer. 1996; 73:972–978.36. Sun Y, Liu M, Yang B, Li B, Lu J. Role of siRNA silencing of MMP-2 gene on invasion and growth of laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2008; 265:1385–1391.37. Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001; 276:7549–7558.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association Analysis of MUC5AC Promoter Polymorphism with Asthma
- Leukotriene C4 synthase promoter polymorphism in aspirin - induced asthma
- MMP-1 promoter polymorphism in Korean with generalized aggressive periodontitis
- Functional Polymorphism in the Promoter Region of Matrix Metalloproteinase-9 is Strongly Associated with Acute Myocardial Infarction
- Transforming Growth Factor-beta1 Promoter Polymorphism in Childhood Asthma