Korean J Hematol.
2009 Jun;44(2):82-91. 10.5045/kjh.2009.44.2.82.
The Effect of an Ectopic Overexpression of MnSOD in Mouse Hematopoietic Stem Cells
- Affiliations
-
- 1Department of Internal Medicine, Wonkwang University School of Medicine Iksan, Korea. mrpark21@wonkwang.ac.kr
- 2Department of Radiation Oncology, University of Pittsburgh Cancer Institute, Pittsburgh, USA.
- KMID: 2252152
- DOI: http://doi.org/10.5045/kjh.2009.44.2.82
Abstract
-
BACKGROUND: Intracellular reactive oxygen species (ROS) have dual effects depending on their cellular level. ROS act as secondary messengers at a low concentration, although ROS exhaust the hematopoietic stem cell (HSC) compartment at a higher oxidized state. So, we investigated whether maintaining a low level of ROS could preserve the hematopoietic stem cell function according to the MnSOD over expression.
METHODS
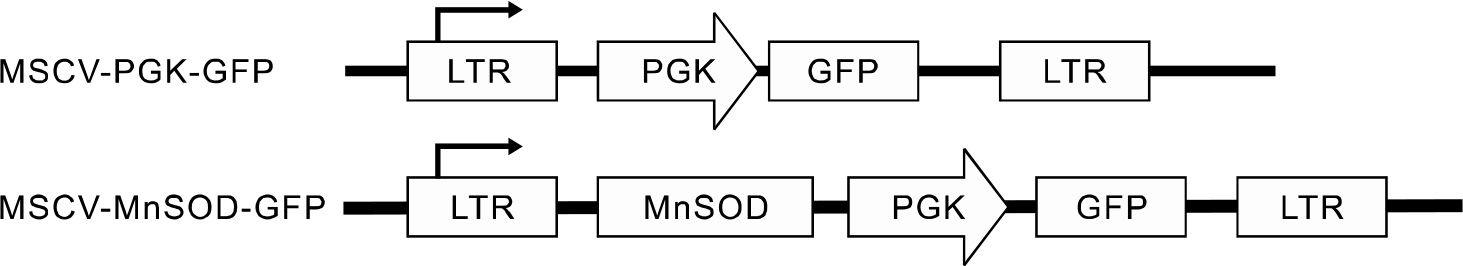
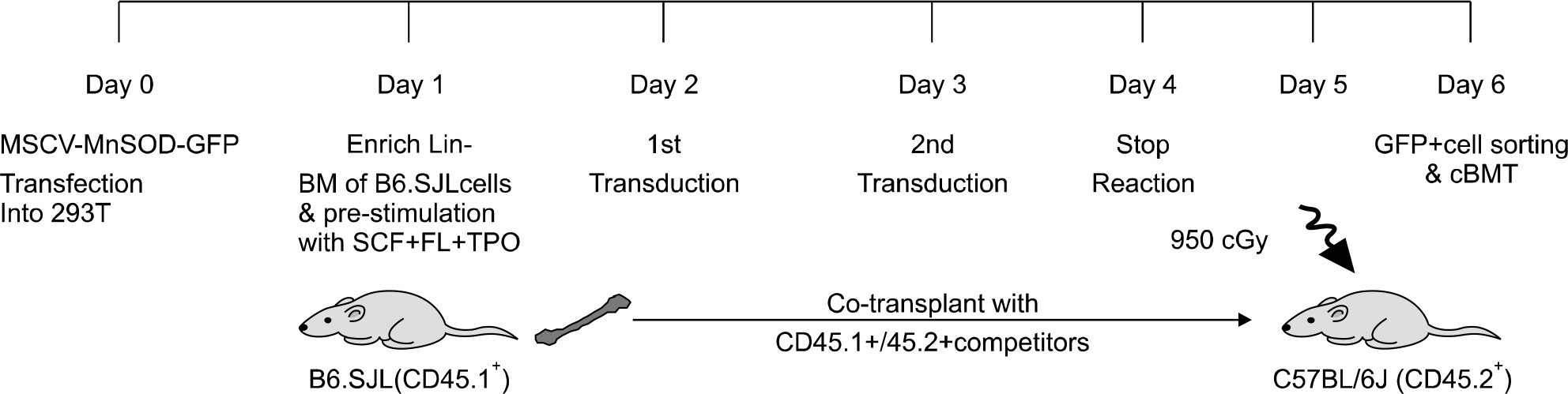
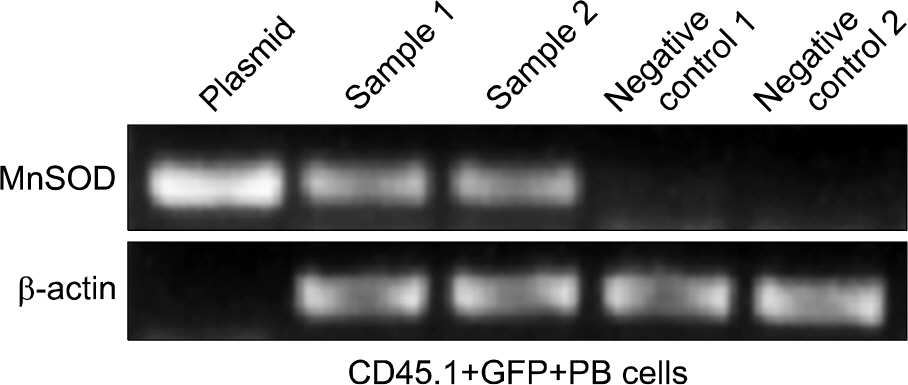
Human MnSOD cDNA was introduced into mouse HSCs and progenitor cells by using a MSCV-PGK-GFP retrovirus. The hematopoietic function of over-expressing MnSOD was evaluated in vitro on a colony-forming cell assay and in vivo in a competitive transplantation model. MnSOD-transduced, lineage negative, GFP+B6.SJL (CD45.1+) mouse bone marrow cells were transplanted into lethally irradiated C57BL/6J (CD45.2+) mice in competition with CD45.1/45.2 double positive bone marrow mononuclear cells. We also measured the basal mRNA levels of antioxidants, including MnSOD, catalase and cellular glutathione peroxidase (GPx1), of C57BL/6J HSCs.
RESULTS
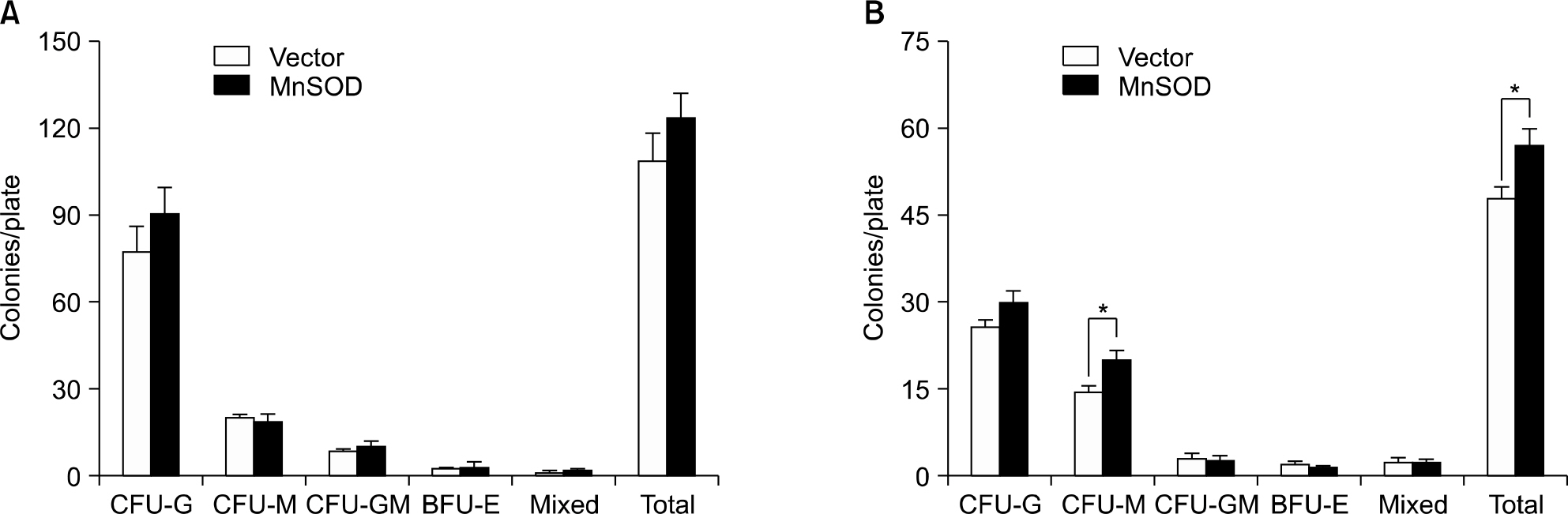
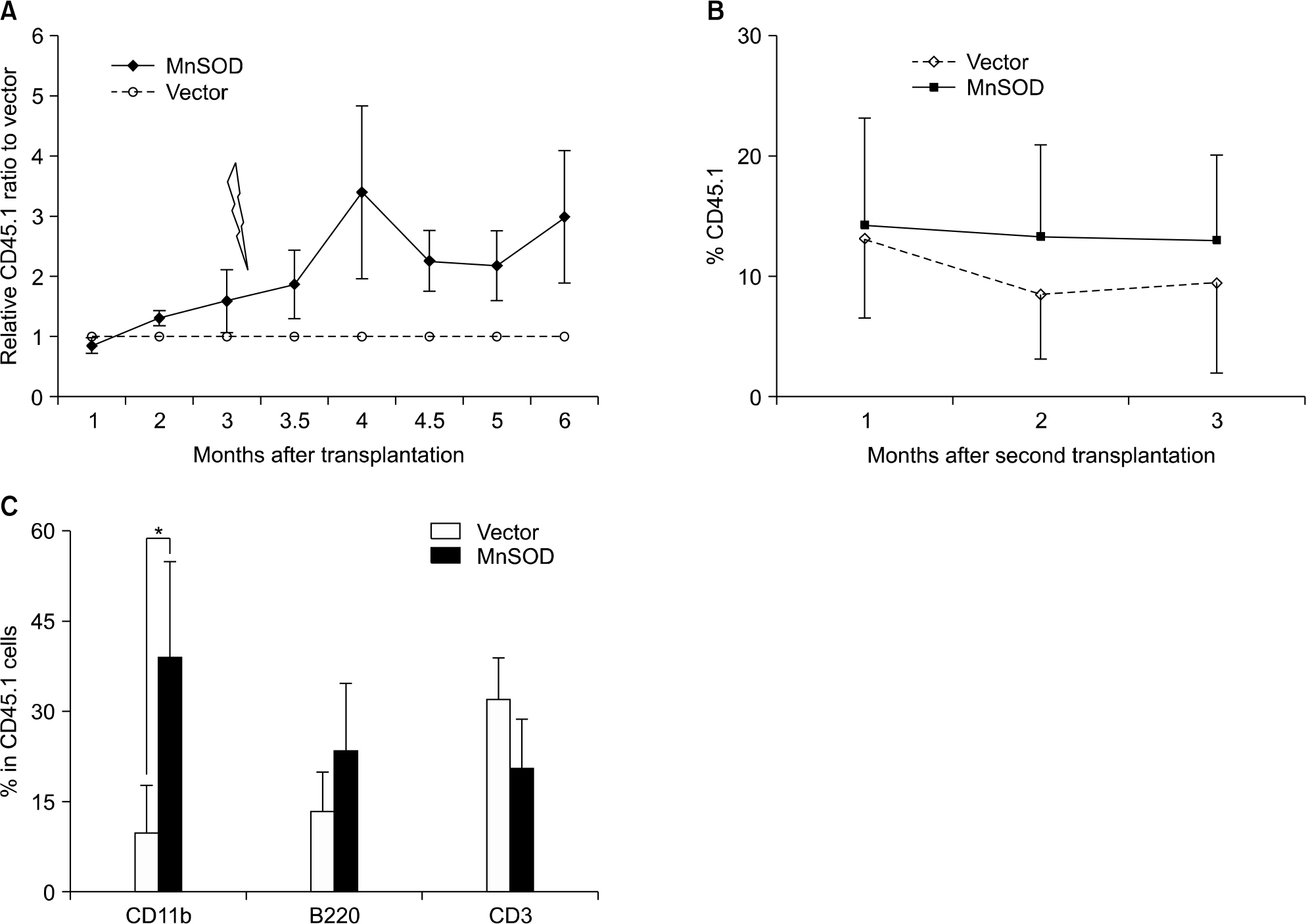
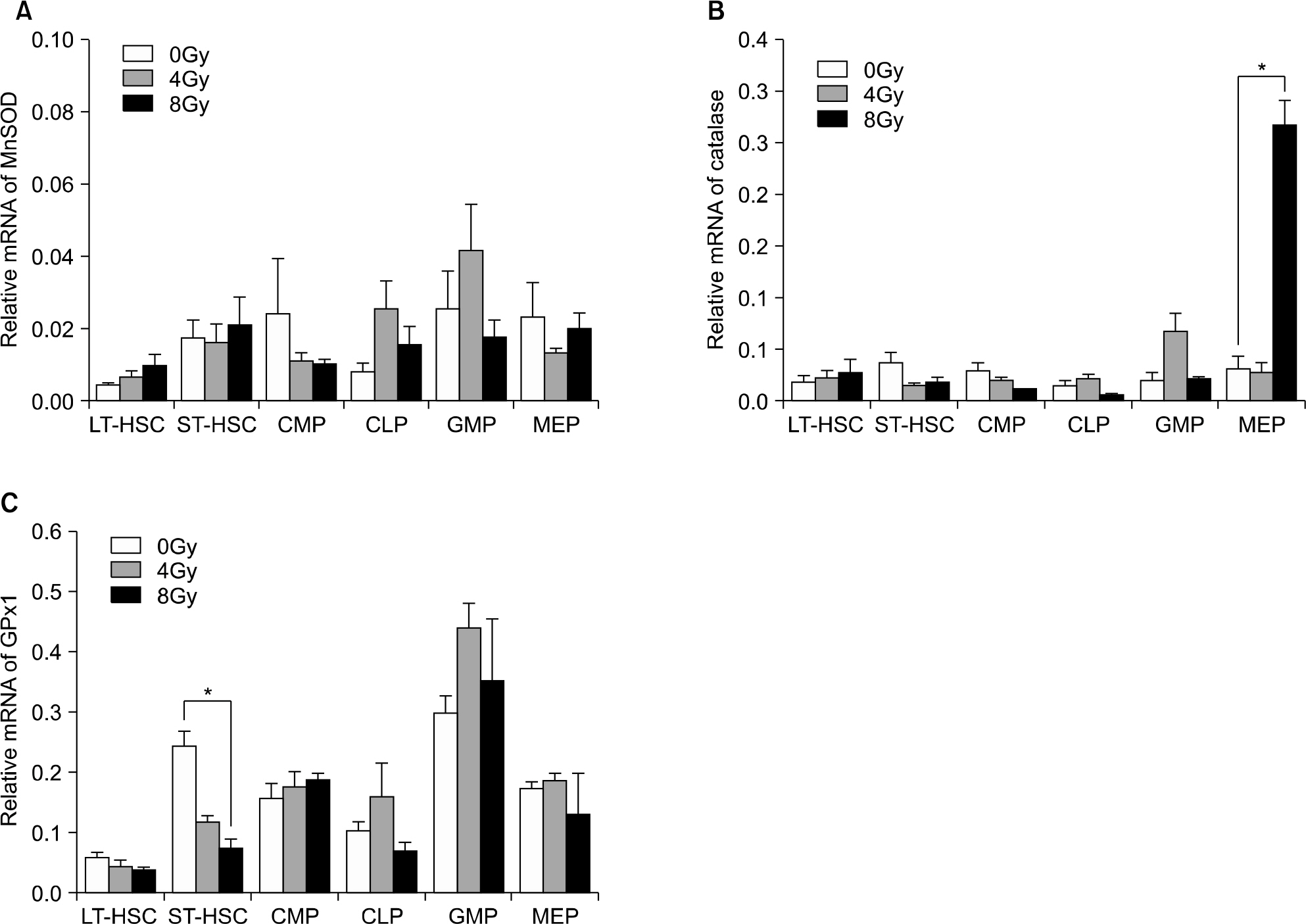
On the colony-forming cell assay, a MnSOD over expression significantly preserved the CFU-M with irradiation as compared with the mice without irradiation. HSCs with an MnSOD over expression showed a tendency for higher engraftment ability on the competitive transplantation assay even after 200 cGy re-irradiation, and we observed a significantly higher myeloid differentiation potential after the second serial transplantation. The basal mRNA levels of MnSOD and catalase were less than 1~2% and 2~5%, respectively, in the long-term and short-term HSCs, respectively, and these cells didn't activate in spite of radiation stress.
CONCLUSION
These results show that only an over expression of MnSOD without downstream catalase activation can not augment the mouse hematopoietic stem cell repopulation activity.
MeSH Terms
-
Animals
Antioxidants
Bone Marrow
Bone Marrow Cells
Catalase
DNA, Complementary
Glutathione Peroxidase
Hematopoietic Stem Cells
Humans
Mice
Reactive Oxygen Species
Retroviridae
RNA, Messenger
Stem Cells
Superoxide Dismutase
Transplants
Antioxidants
Catalase
DNA, Complementary
Glutathione Peroxidase
RNA, Messenger
Reactive Oxygen Species
Superoxide Dismutase
Figure
Reference
-
References
1. Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med. 1999; 27:936–44.
Article2. Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006; 12:4427–43.
Article3. Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004; 337:1–13.
Article4. Noble M, Mayer-Pröschel M, Pröschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005; 7:1456–67.
Article5. Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004; 431:997–1002.
Article6. Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007; 128:325–39.7. Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006; 12:446–51.
Article8. Piccoli C, D'Aprile A, Ripoli M, et al. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem Biophys Res Commun. 2007; 353:965–72.
Article9. Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007; 110:3056–63.
Article10. Kinnula VL, Crapo JD. Superoxide dismutases in malignant cells and human cancers. Free Radic Biol Med. 2004; 36:718–44.11. Li N, Oberley TD. Modulation of antioxidant enzymes, reactive oxygen species, and glutathione levels in manganese superoxide dismutase-over expressing NIH/3T3 fibroblasts during the cell cycle. J Cell Physiol. 1998; 177:148–60.12. Kim A, Zhong W, Oberley TD. Reversible modulation of cell cycle kinetics in NIH/3T3 mouse fibroblasts by inducible over expression of mitochondrial manganese superoxide dismutase. Antioxid Redox Signal. 2004; 6:489–500.13. Epperly MW, Bernarding M, Gretton J, Jefferson M, Nie S, Greenberger JS. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-alpha, IL-3 withdrawal, and ionizing radiation. Exp Hematol. 2003; 31:465–74.14. Epperly MW, Osipov AN, Martin I, et al. Ascorbate as a “redox sensor” and protector against irradiation-induced oxidative stress in 32D CL 3 hematopoietic cells and subclones over expressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004; 58:851–61.15. Southgate TD, Sheard V, Milsom MD, et al. Radioprotective gene therapy through retroviral expression of manganese superoxide dismutase. J Gene Med. 2006; 8:557–65.
Article16. Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995; 11:376–81.
Article17. Lebovitz RM, Zhang H, Vogel H, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996; 93:9782–7.
Article18. Friedman JS, Rebel VI, Derby R, et al. Absence of mitochondrial superoxide dismutase results in a murine hemolytic anemia responsive to therapy with a catalytic antioxidant. J Exp Med. 2001; 193:925–34.
Article19. Zhang Y, Smith BJ, Oberley LW. Enzymatic activity is necessary for the tumor-suppressive effects of MnSOD. Antioxid Redox Signal. 2006; 8:1283–93.
Article20. Zhang HJ, Yan T, Oberley TD, Oberley LW. Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype. Cancer Res. 1999; 59:6276–83.21. Church SL, Grant JW, Ridnour LA, et al. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci USA. 1993; 90:3113–7.
Article22. Liu R, Oberley TD, Oberley LW. Transfection and expression of MnSOD cDNA decreases tumor malignancy of human oral squamous carcinoma SCC-25 cells. Hum Gene Ther. 1997; 8:585–95.
Article23. Li N, Oberley TD, Oberley LW, Zhong W. Overexpression of manganese superoxide dismutase in DU145 human prostate carcinoma cells has multiple effects on cell phenotype. Prostate. 1998; 35:221–33.
Article25. Rodriguez AM, Carrico PM, Mazurkiewicz JE, Me-léndez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H (2)O(2). Free Radic Biol Med. 2000; 29:801–13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Splitomicin, a SIRT1 Inhibitor, Enhances Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- Hematopoietic Stem Cell Transplantation
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Enforced Expression of BMI-1 in Postnatal Human CD34+ Cells Promotes Erythroid Differentiation
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells