Korean J Hematol.
2009 Dec;44(4):212-219. 10.5045/kjh.2009.44.4.212.
Effect of Etanercept on Steroid Refractory Graft-versus-host Disease in Children
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Chungnam National University, Daejeon, Korea. sunyoung@cnuh.co.kr
- KMID: 2252099
- DOI: http://doi.org/10.5045/kjh.2009.44.4.212
Abstract
- BACKGROUND
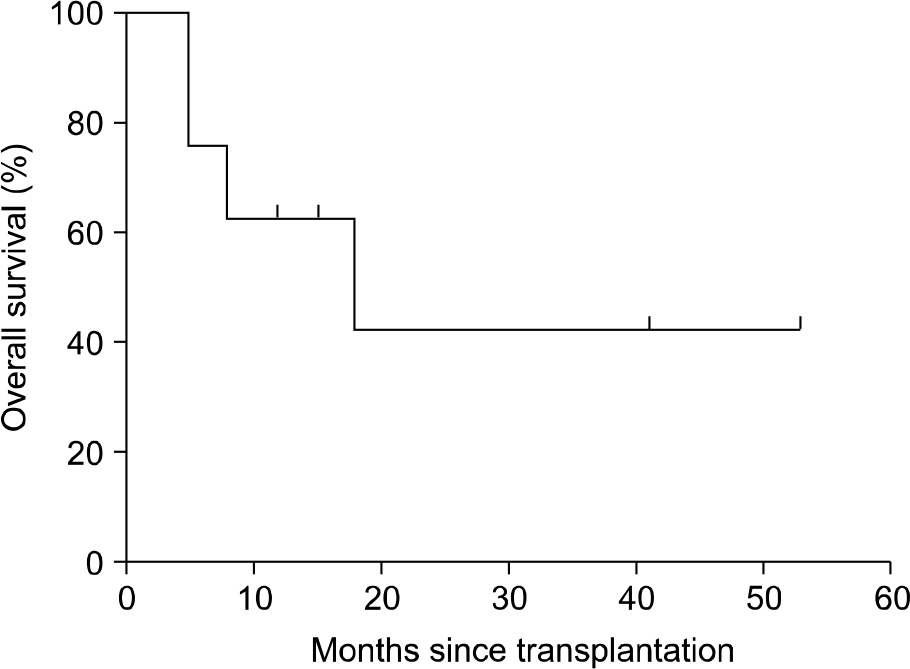
Etanercept is a recombinant human soluble tumor necrosis factor-alpha (TNF-alpha) receptor fusion protein that inhibits TNF-alpha, a major mediator in the pathogenesis of graft-versus-host disease (GVHD). The purpose of our study was to evaluate the safety and efficacy of etanercept therapy in children with steroid-refractory acute GVHD (aGVHD) (n=5) and chronic GVHD (cGVHD) (n=3). METHODS: Five males and 3 females were enrolled and their median age was 14.4 years (range, 2.1~18.8). Etanercept 0.4 mg/kg per dose (maximum dose, 25 mg) was given subcutaneously twice weekly for 4 weeks followed by 0.4 mg/kg per dose (maximum dose, 25 mg) weekly for 4 weeks. At the time of initiation of etanercept, 5 patients had aGVHD grade III to IV (III=4, IV=1) and 3 patients had moderate to severe cGVHD (moderate=1, severe=2). RESULTS: Overall, 6 of 8 patients (75%) responded to the treatment with etanercept, including 5 patients with aGVHD [n=3 complete response (CR), n=2 partial response (PR)] and 1 patient with cGVHD [n=1 PR, n=2 no response (NR)]. Clinical responses were most commonly seen in patients with refractory gut aGVHD. CMV reactivation occurred in 2 patients, bacterial infection in 1 patient, and fungal infection in 1 patient. CONCLUSION: Our preliminary data indicate that etanercept is well tolerated and can induce a high response rate in patients with steroid refractory aGVHD, particularly in the setting of intestinal involvement.
MeSH Terms
Figure
Reference
-
References
1. Deeg HJ. How I treat refractory acute GVHD. Blood. 2007; 109:4119–26.
Article2. Kim SS. Treatment options in steroid-refractory acute graft-versus-host disease following hematopoietic stem cell transplantation. Ann Pharmacother. 2007; 41:1436–44.
Article3. Nogueira MC, Azevedo AM, Pereira SC, et al. Antitumor necrosis factor-α for the treatment of steroid-refractory acute graft-versus-host disease. Braz J Med Biol Res. 2007; 40:1623–9.4. Ritchie D, Seconi J, Wood C, Walton J, Watt V. Prospective monitoring of tumor necrosis factor alpha and interferon gamma to predict the onset of acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005; 11:706–12.5. Uberti JP, Ayash L, Ratanatharathorn V, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005; 11:680–7.
Article6. Wolff D, Roessler V, Steiner B, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant. 2005; 35:1003–10.
Article7. Chiang KY, Abhyankar S, Bridges K, Godder K, Henslee-Downey JP. Recombinant human tumor necrosis factor receptor fusion protein as complementary treatment for chronic graft-versus-host disease. Transplantation. 2002; 73:665–7.8. Busca A, Locatelli F, Marmont F, Ceretto C, Falda H. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007; 82:45–52.
Article9. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995; 15:825–8.10. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11:945–56.11. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. Clin Infect Dis. 2004; 39(Suppl 1):S53–5.
Article12. Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990; 76:1464–72.
Article13. MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002; 8:387–94.
Article14. Kobbe G, Schneider P, Rohr U, et al. Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse anti-TNFalpha antibody. Bone Marrow Transplant. 2001; 28:47–9.15. Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004; 104:649–54.16. Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999; 116:593–601.
Article17. Andolina M, Rabusin M, Maximova N, Di Leo G. Etanercept in graft-versus-host disease. Bone Marrow Transplant. 2000; 26:929.
Article18. Coriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-α blockade for the treatment of acute GVHD. Blood. 2004; 104:649–54.19. Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004; 89:1352–9.20. Holler E, Kolb HJ, Möller A, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990; 75:1011–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Etanercept for steroid-refractory acute graft versus host disease following allogeneic hematopoietic stem cell transplantation
- Acute Cutaneous Graft-Versus-Host Reaction
- Fecal Microbiota Transplantation for Treating Steroid-Refractory Acute Graft-versus-Host Disease of the Gut
- Chronic Graft Versus Host Disease
- Therapeutic Effects of 0.03% Tacrolimus Eye Drops for Chronic Ocular Graft-Versus-Host Disease