J Rheum Dis.
2013 Apr;20(2):74-82. 10.4078/jrd.2013.20.2.74.
Phosphoinositide 3-kinase (PI3K) as a New Therapeutic Target for Rheumatoid Arthritis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea. kimhaerim@kuh.ac.kr
- KMID: 2223030
- DOI: http://doi.org/10.4078/jrd.2013.20.2.74
Abstract
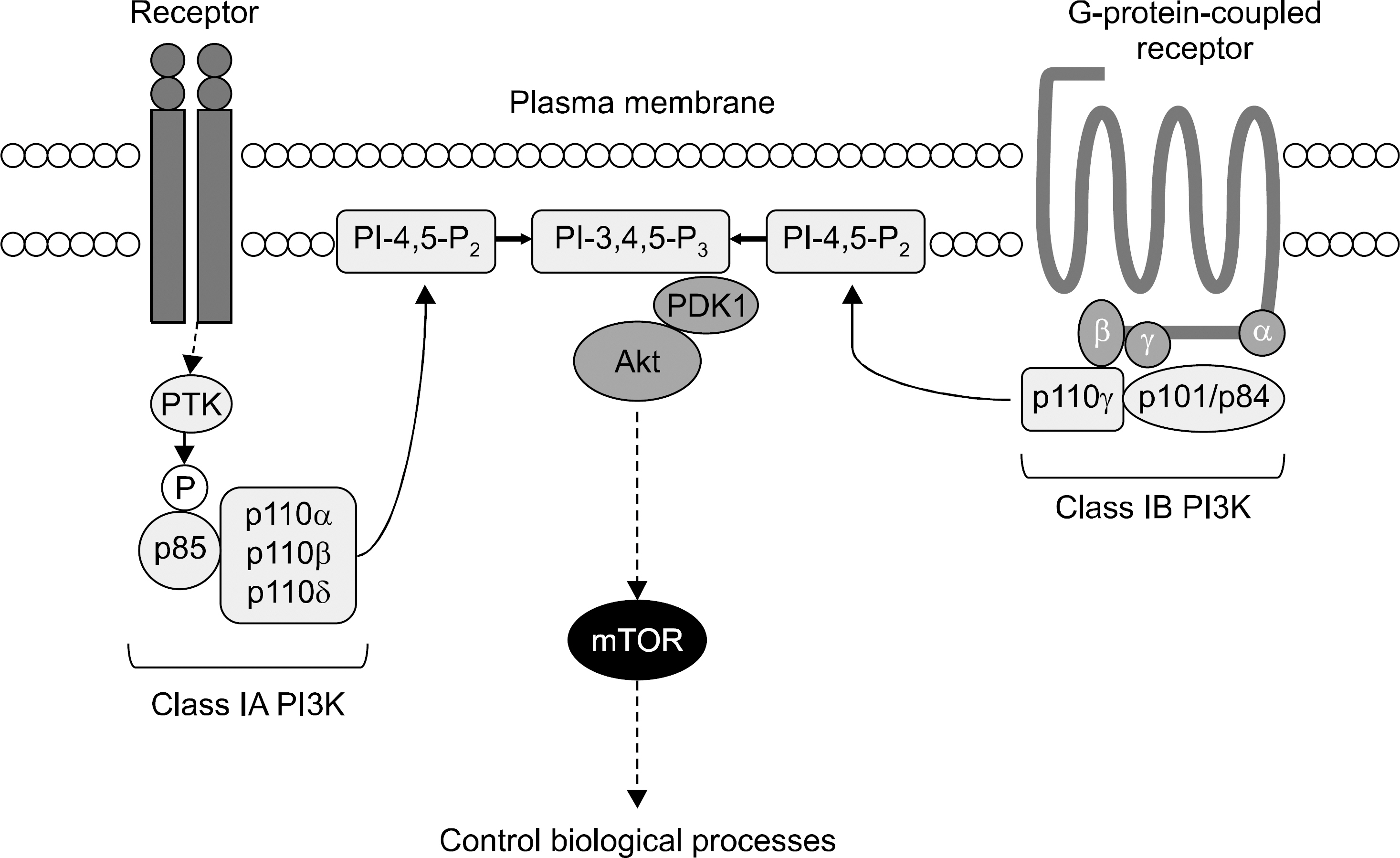
- Dysregulated activation of immune and synovial cells and their reciprocal action play a key role in the pathogenesis of rheumatoid arthritis (RA). Various signal transduction molecules regulate cellular responses and small molecular inhibitors targeting the signal molecules, such as Janus kinase (JAK) and spleen tyrosine kinase (Syk) inhibitors, which have been developed for treating RA. Phosphoinositide 3-kinase (PI3K) is one of the signal molecules, which regulates innate and adaptive immune systems and is over-expressed in RA. PI3Ks phosphorylate phosphoinositide-4,5-bisphosphate (PI-4,5-P2) generates phosphoinositide-3,4,5-triphosphate (PI-3,4,5-P3) at the cell membrane. PI3Ks are divided into class I, II and III. Two catalytic subunits, p110gamma and p110delta of PI3K, modulate cellular development, differentiation, proliferation, migration, cytokine synthesis and antibody production in both innate and adaptive immune systems. In RA synovium and synovial fibroblasts, the expression of p110gamma and p110delta is increased, and their up-regulation results in the abnormal activation of cellular immune responses. In preclinical animal models for RA, genetic deletion of p110gamma and p110delta and selective inhibitors decrease the clinical arthritis score, synovial inflammation, cellular infiltration, bone and cartilage erosion and osteoclast activity. There is a synergistic effect for controlling arthritis by dual inhibition of PI3Kgamma and PI3Kdelta. Through reviewing the function of PI3K in the immune system and the effect of PI3K inhibition in preclinical arthritis animal models, we can expect the PI3K inhibition as a new therapeutic target for treatment of RA.
MeSH Terms
-
Antibody Formation
Arthritis
Arthritis, Rheumatoid
Cartilage
Catalytic Domain
Cell Membrane
Fibroblasts
Immune System
Immunity, Cellular
Inflammation
Intracellular Signaling Peptides and Proteins
Models, Animal
Osteoclasts
Phosphatidylinositols
Phosphotransferases
Protein-Tyrosine Kinases
Signal Transduction
Spleen
Synovial Membrane
Up-Regulation
Intracellular Signaling Peptides and Proteins
Phosphatidylinositols
Phosphotransferases
Protein-Tyrosine Kinases
Figure
Reference
-
References
1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010; 376:1094–108.
Article2. Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac'h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012; 39:222–8.
Article3. Keystone EC, Smolen J, van Riel P. Developing an effective treatment algorithm for rheumatoid arthritis. Rheumatology (Oxford). 2012; 51(Suppl 5):v48–54.
Article4. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–81.
Article5. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012; 64:625–39.
Article6. Rubbert-Roth A. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology (Oxford). 2001; 51(Suppl 5):v38–47.
Article7. Fleischmann R. Novel small-molecular therapeutics for rheumatoid arthritis. Curr Opin Rheumatol. 2012; 24:335–41.
Article8. Cohen S, Fleischmann R. Kinase inhibitors: a new ap-proach to rheumatoid arthritis treatment. Curr Opin Rheumatol. 2010; 22:330–5.
Article9. Norman P. Selective JAK1 inhibitor and selective Tyk2 inhibitor patents. Expert Opin Ther Pat. 2012; 22:1233–49.
Article10. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002; 296:1655–7.
Article11. Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998; 18:1379–87.12. Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obli-gate p85-p110 heterodimers. Proc Natl Acad Sci U S A. 2007; 104:7809–14.
Article13. Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002; 14:7–18.
Article14. Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, et al. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997; 89:105–14.15. Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008; 1784; 159–85.16. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007; 129:1261–74.
Article17. Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001; 59:3–16.
Article18. Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM. Structure, function, and biology of SHIP proteins. Genes Dev. 2000; 14:505–20.
Article19. Wishart MJ, Dixon JE. PTEN and myotubularin phospha-tases: from 3-phosphoinositide dephosphorylation to disease. Trends Cell Biol. 2002; 12:579–85.
Article20. Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999; 274:10963–8.21. Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002; 13:169–72.22. Barber DF, Bartolomé A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005; 11:933–5.23. Comerford I, Litchfield W, Kara E, McColl SR. PI3Kγ drives priming and survival of autoreactive CD4(+) T cells during experimental autoimmune encephalomyelitis. PLoS One. 2012; 7:e45095.
Article24. Ferrandi C, Ardissone V, Ferro P, Rückle T, Zaratin P, Ammannati E, et al. Phosphoinositide 3-kinase gamma inhibition plays a crucial role in early steps of inflammation by blocking neutrophil recruitment. J Pharmacol Exp Ther. 2007; 322:923–30.25. Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007; 7:191–201.26. Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000; 287:1040–6.27. Webb LM, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005; 175:2783–7.28. Swat W, Montgrain V, Doggett TA, Douangpanya J, Puri K, Vermi W, et al. Essential role of PI3Kdelta and PI3Kgamma in thymocyte survival. Blood. 2006; 107:2415–22.29. Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, et al. Sjögren's syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci USA. 2006; 103:16882–7.30. Okkenhaug K, Patton DT, Bilancio A, Garçon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006; 177:5122–8.31. Locke NR, Patterson SJ, Hamilton MJ, Sly LM, Krystal G, Levings MK. SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol. 2009; 183:975–83.
Article32. Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006; 177:6598–602.33. Puri KD, Gold MR. Selective inhibitors of phosphoinositide 3-kinase delta: modulators of B-cell function with potential for treating autoimmune inflammatory diseases and B-cell malignancies. Front Immunol. 2012; 3:256.
Article34. Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002; 297:1031–4.35. Sujobert P, Bardet V, Cornillet-Lefebvre P, Hayflick JS, Prie N, Verdier F, et al. Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood. 2005; 106:1063–6.36. Bilancio A, Okkenhaug K, Camps M, Emery JL, Ruckle T, Rommel C, et al. Key role of the p110delta isoform of PI3K in B-cell antigen and IL-4 receptor signaling: comparative analysis of genetic and pharmacologic inter-ference with p110delta function in B cells. Blood. 2006; 107:642–50.37. Zhang TT, Okkenhaug K, Nashed BF, Puri KD, Knight ZA, Shokat KM, et al. Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. J Allergy Clin Immunol. 2008; 122:811–9.38. Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, et al. Regulation of class- switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006; 25:545–57.39. Ji H, Rintelen F, Waltzinger C, Bertschy Meier D, Bilancio A, Pearce W, et al. Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood. 2007; 110:2940–7.40. Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in che-moattractant-mediated signal transduction. Science. 2000; 287:1046–9.41. Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005; 106:1432–40.
Article42. Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, Davidson K, et al. PI3Kβ plays a critical role in neutrophil activation by immune complexes. Sci Signal. 2011; 4:ra23.
Article43. Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006; 20:455–65.44. Wymann MP, Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol. 2005; 17:141–9.
Article45. Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006; 80:1491–9.46. Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, et al. PI(3)Kgamma has an important con-text-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007; 9:86–91.47. Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J. 2004; 23:3505–15.48. Berod L, Heinemann C, Heink S, Escher A, Stadelmann C, Drube S, et al. PI3Kγ deficiency delays the onset of experimental autoimmune encephalomyelitis and amelio-rates its clinical outcome. Eur J Immunol. 2011; 41:833–44.
Article49. Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarla-gadda M, Vanhaesebroeck B, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008; 14:565–73.
Article50. Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005; 11:936–43.51. Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol. 2008; 38:1215–24.52. Gruen M, Rose C, König C, Gajda M, Wetzker R, Bräuer R. Loss of phosphoinositide 3-kinase gamma decreases migration and activation of phagocytes but not T cell activation in antigen-induced arthritis. BMC Musculoskelet Disord. 2010; 11:63.
Article53. Hayer S, Pundt N, Peters MA, Wunrau C, Kühnel I, Neugebauer K, et al. PI3Kgamma regulates cartilage damage in chronic inflammatory arthritis. FASEB J. 2009; 23:4288–98.54. Toyama S, Tamura N, Haruta K, Karakida T, Mori S, Watanabe T, et al. Inhibitory effects of ZSTK474, a novel phosphoinositide 3-kinase inhibitor, on osteoclasts and collagen-induced arthritis in mice. Arthritis Res Ther. 2010; 12:R92.
Article55. Bartok B, Boyle DL, Liu Y, Ren P, Ball ST, Bugbee WD, et al. PI3 kinase δ is a key regulator of synoviocyte function in rheumatoid arthritis. Am J Pathol. 2012; 180:1906–16.
Article56. Soond DR, Bj⊘rgo E, Moltu K, Dale VQ, Patton DT, Torgersen KM, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010; 115:2203–13.57. Banham-Hall E, Clatworthy MR, Okkenhaug K. The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases. Open Rheumatol J. 2012; 6:245–58.
Article58. Meadows SA, Vega F, Kashishian A, Johnson D, Diehl V, Miller LL, et al. PI3Kδ inhibitor, GS-1101 (CAL- 101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood. 2012; 119:1897–900.59. Norman P. Selective PI3Kδ inhibitors, a review of the patent literature. Expert Opin Ther Pat. 2011; 21:1773–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PBT-6, a Novel PI3KC2γ Inhibitor in Rheumatoid Arthritis
- Regulation of Interleukin-17 Production in Patients with Rheumatoid Arthritis by Phosphoinositide 3-kinase (PI3K)/ Akt and Nuclear Factor KappaB (NF-kappaB) Dependent Signal Transduction Pathway
- Cytokines in rheumatoid arthritis
- Clinical significance of rheumatoid factor in juvenile rheumatoid arthritis
- Homo-Genius: Homocitrulline Can Be a Better Target than Citrulline as a Biomarker for Rheumatoid Arthritis?