J Korean Ophthalmol Soc.
2016 May;57(5):734-741. 10.3341/jkos.2016.57.5.734.
Comparison of Therapeutic Effects of 3% Diquafosol Tetrasodium with Aging in Dry Eye
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea. jongsool@pusan.ac.kr
- 2Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Gyeongsang National University Changwon Hospital, Changwon, Korea.
- KMID: 2212715
- DOI: http://doi.org/10.3341/jkos.2016.57.5.734
Abstract
- PURPOSE
The aim of this study was to evaluate the clinical effect of 3% diquafosol in dry eye patients aged around 60 years.
METHODS
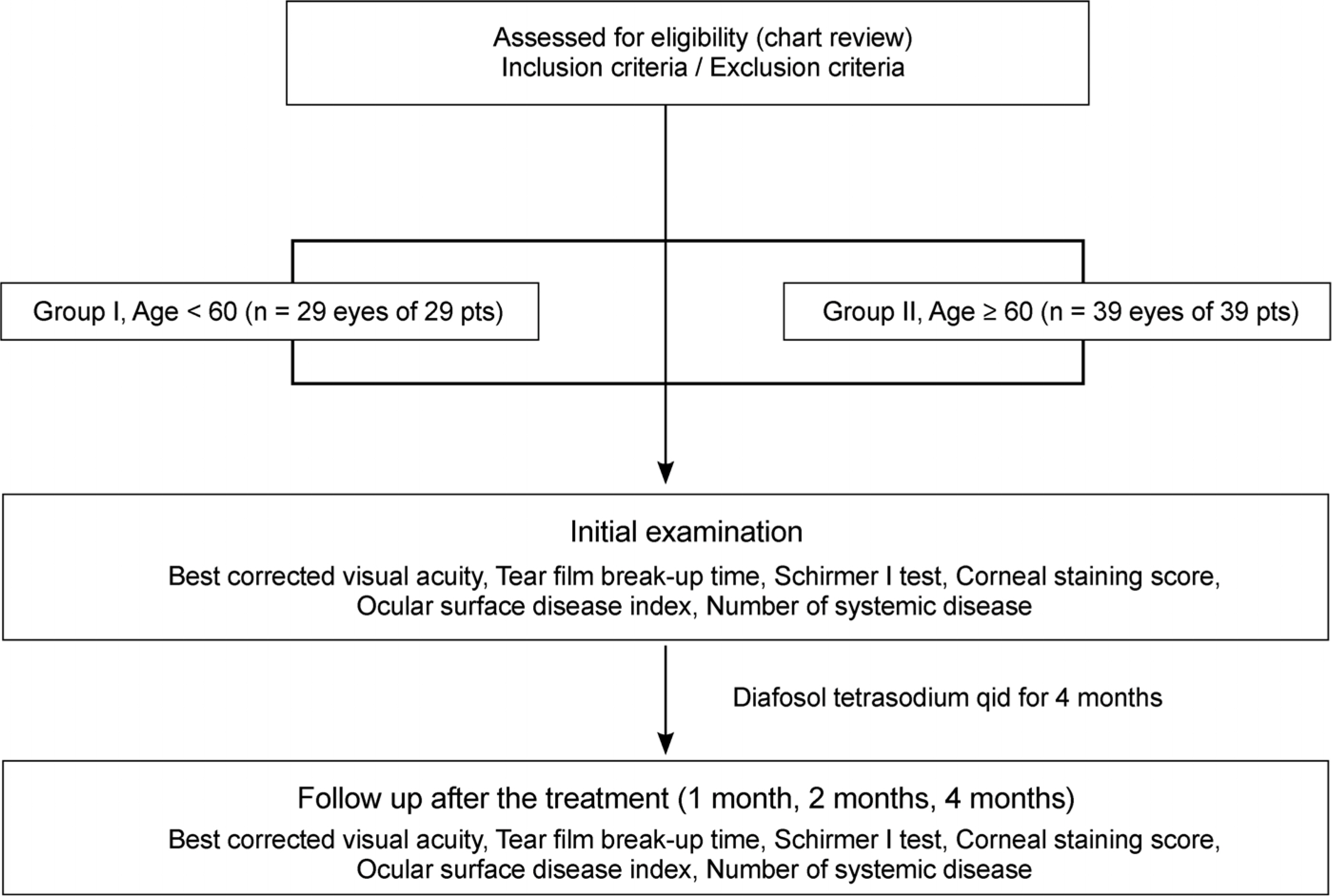
In total, 68 patients with dry eye syndrome were divided by age into 2 groups, Group I (29 patients, 29 eyes) under the age of 60 years and Group II (39 patients, 39 eyes) over the age of 60 years. To evaluate the effectiveness of 3% diquafosol, we measured the tear film break-up time (tBUT), performed the Schirmer I test, and used the corneal staining score as an objective indicator and the ocular surface disease index (OSDI) score as a subjective indicator at initial visit, 1 month, 2 months, and 4 months.
RESULTS
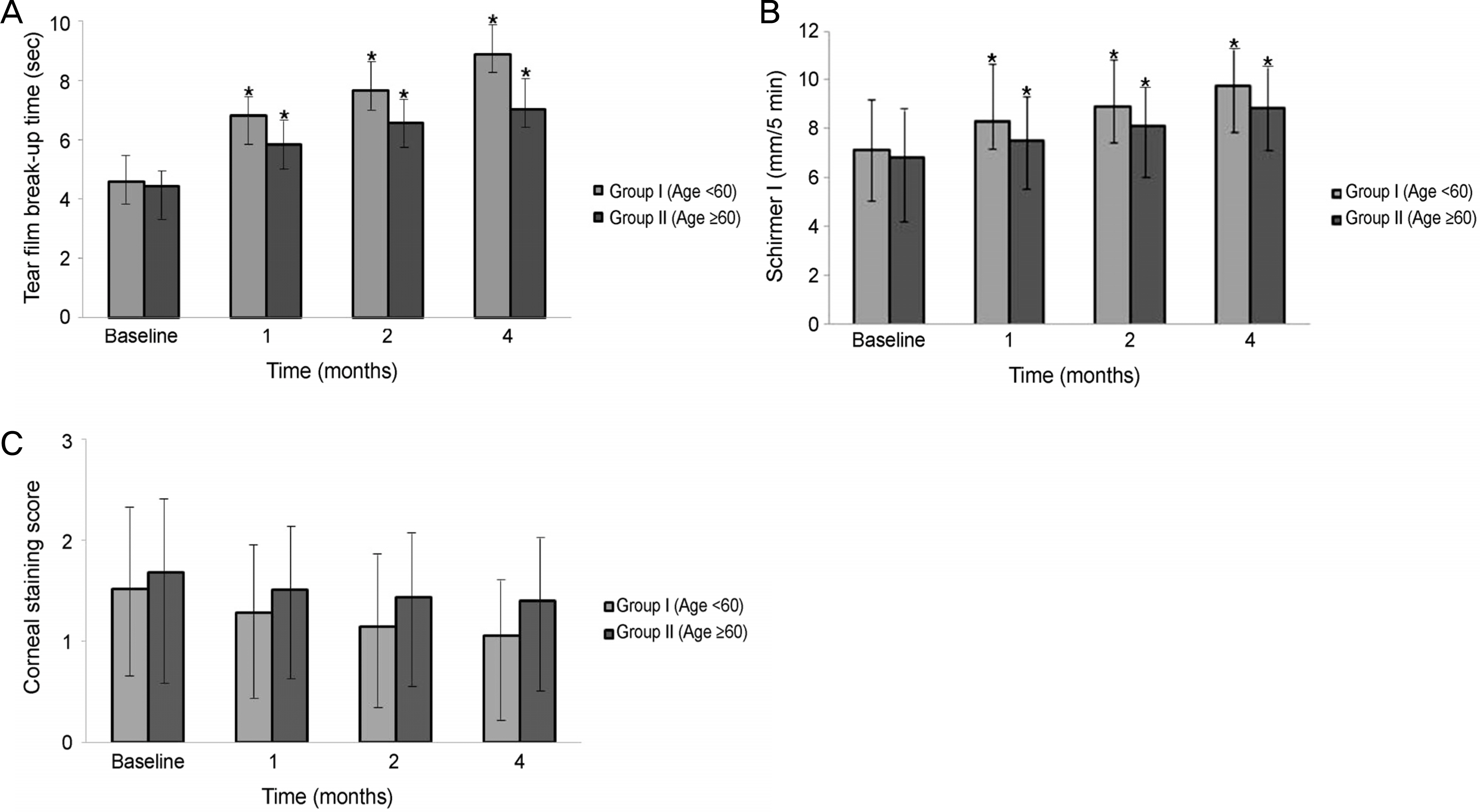
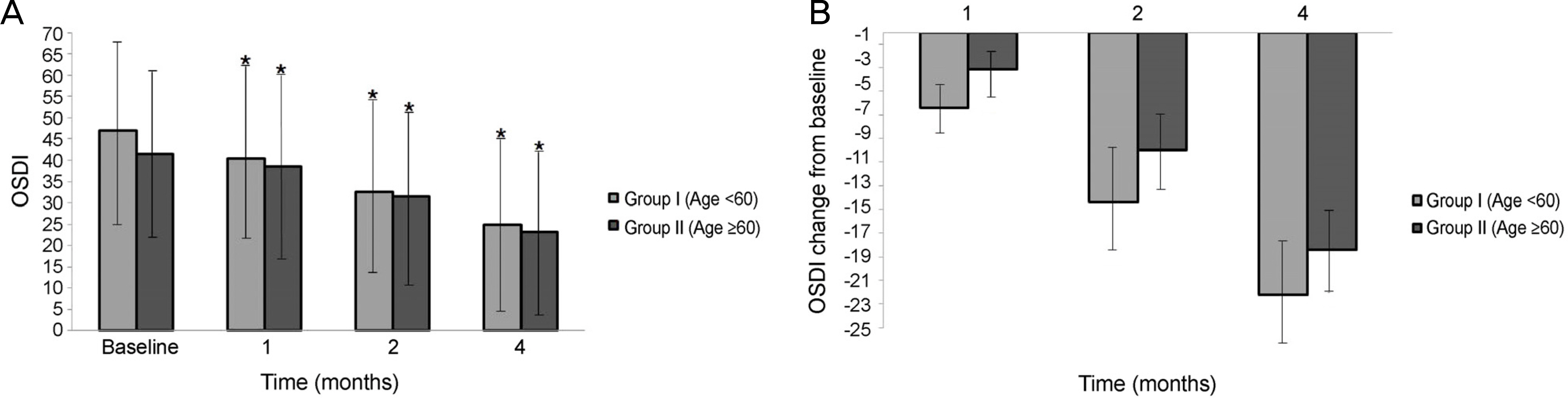
Significant improvements in the tear film break-up time, Schirmer I test, and OSDI were observed at 1,2, and 4 months after treatment with 3% diquafosol tetrasodium in both dry eye groups, but significant difference in the corenal staining score were not observed (p > 0.05). There were statistically significant improvement between the 2 age groups in the tBUT at 1 month (p = 0.012), 2 months (p = 0.005), and 4 months (p = 0.005), and improvements in the Schirmer I test between the 2 age groups at 1 month (p = 0.015), 2 months (p = 0.005), and 4 months (p = 0.005) were also observed. But, there was no significant difference in the corneal staining score and OSDI score between the 2 groups at 1, 2, and 4 months (p > 0.05).
CONCLUSIONS
Topical 3% diquafosol tetrasodium administration was shown to be an effective treatment to improve clinical symptoms and objective indicators in dry eye patients regardless of age, showing significant improvements in tBUT and the Schirmer I test under the age of 60.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Use of Keratography to Study Changes on the Ocular Surface after Absorbable Plug Insertion
Hee Jong Shin, Chang-Hyun Park, Kyung Sun Na, Hyun Seung Kim
J Korean Ophthalmol Soc. 2018;59(1):17-22. doi: 10.3341/jkos.2018.59.1.17.Clinical Significance of Computerized Videokeratoscopic Indices for Dry Eye
Jong-Ha Lee, Min-Hwan Kim, Byung-Yi Ko
J Korean Ophthalmol Soc. 2019;60(7):627-634. doi: 10.3341/jkos.2019.60.7.627.
Reference
-
References
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007; 5:75–92.2. Paulsen AJ. Cruickshanks KJ. Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors and health-related quality of life. Am J Ophthalmol. 2014; 157:799–806.
Article3. Damasceno RW. Oksaki MH. Dantas PE. Belfort R Jr.Involutional entropion and ectropion of the lower eyelid: prevalence and associated risk factors in the elderly population. Ophthal Plast Reconstr Surg. 2011; 27:317–20.
Article4. Sullivan DA. Jensen RV. Suzuki T. Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Mol Vis. 2009; 15:1553–72.5. Tsubota K. Kawashima M. Inaba T, et al. The antiaging approach for the treatment of dry eye. Cornea. 2012; 31:1. S3–8.
Article6. Foulks GN. Pharmacological management of dry eye in the elderly patient. Drugs Aging. 2008; 25:105–18.
Article7. Fujihara T. Murakami T. Fujita H, et al. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42:96–100.8. Matsumoto Y. Ohashi Y. Watanabe H, et al. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012; 119:1954–60.
Article9. Kamiya K. Nakanishi M. Ishii R, et al. Clinical evalution of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: a prospective, randomized, multicenter study. Eye (Lond). 2012; 26:1363–8.10. Takamura E. Tsubota K. Watanabe H, et al. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012; 96:1310–5.
Article11. Kaido M. Uchino M. Kojima T, et al. Effects of diquafosol tetrasodium administration on visual function in short break-up time dry eye. J Ocul Pharmacol Ther. 2013; 29:595–603.
Article12. Shimazaki-Den S. Iseda H. Dogru M, et al. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea. 2013; 32:1120–5.
Article13. Jung HH. Kang YS. Sung MS. Yoon KC. Clinical efficacy of topical 3% diquafosol tetrasodium in short tear film break-up time dry eye. J Korean Ophthalmol Soc. 2015; 56:339–44.
Article14. Lemp MA. Crews LA. Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012; 31:472–8.15. Yoon KC. Im SK. Kim HG. You IC. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea. 2011; 30:972–6.
Article16. Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998; 17:565–96.
Article17. Kaido M. Goto E. Dogru M. Tsubota K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology. 2004; 111:895–900.
Article18. Miller KL. Walt JG. Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010; 128:94–101.
Article19. Yamaguchi M. Nishijima T. Shimazaki J, et al. Clinical usefulness of diquafosol for real-world dry eye patients: a prospective, open-label, non-interventional, observational study. Adv Ther. 2014; 31:1169–81.
Article20. Koh S. Ikeda C. Takai Y, et al. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn J Ophthalmol. 2013; 57:440–6.
Article21. Roszkowska AM. Colosi P. Ferreri FM. Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004; 218:350–5.
Article22. Sharma A. Hindman HB. Aging: a predisposition to dry eyes. J Ophthalmol. 2014; 2014:781683.
Article23. Gong L. Sun X. Ma Z, et al. A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br J Ophthalmol. 2015; 99:903–8.
Article24. Yamaguchi M. Nishijima T. Shimazaki J, et al. Real-world assessment of diquafosol in dry eye patients with risk factors such as contact lens, meibomian gland dysfunction, and conjunctivochalasis: subgroup analysis from a prospective observational study. Clin Ophthalmol. 2015; 9:2251–6.25. Nichols KK. Mitchell GL. Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004; 23:272–85.
Article26. Tomlinson A. Khanal S. Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006; 47:4309–15.
Article27. Zhang X. Chen W. De Paiva CS, et al. Interferon-y exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol Vis Sci. 2011; 52:6279–85.28. Gu Q. Dillon CF. Burt VL. Prescription drug use continues to increase: US. prescription drug data for 2007-2008. NCHS Data Brief. 2010; 42:1–8.
Article29. Somogyi A. Hewson D. Muirhead M. Bochner F. Amiloride disposition in geriatric patients: importance of renal function. Br J Clin Pharmacol. 1990; 29:1–8.
Article30. Zemba M. Papadatu CA. Enache VE. Sârbu LN. Ocular surface in glaucoma patients with topical treatment. Oftalmologia. 2011; 55:94–98.31. Mangoni AA. Jackson SH. Age related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004; 57:6–14.32. Koh S. Maeda N. Ikeda C, et al. Effect of diquafosol ophthalmic solution on the optical quality of the eyes in patients with aqueous-deficient dry eye. Acta Ophthalmol. 2014; 92:e671–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Efficacy of Topical 3% Diquafosol Tetrasodium in Short Tear Film Break-Up Time Dry Eye
- Comparison of cytotoxicities and wound healing effects of diquafosol tetrasodium and hyaluronic acid on human corneal epithelial cells
- Clinical Efficacy of Topical Diquafosol Tetrasodium after Laser Epithelial Keratomileusis
- Effect of 3% Diquafosol Tetrasodium on Tear Film Stability after Laser-assisted in situ Keratomileusis
- Changes in Tear Volume after 3% Diquafosol Treatment in Patients with Dry Eye Syndrome: An Anterior Segment Spectral-domain Optical Coherence Tomography Study