J Korean Surg Soc.
2010 Nov;79(5):317-325. 10.4174/jkss.2010.79.5.317.
Immunomodulatory Effects of Mesenchymal Stem Cells in a Murine Model of TNBS-Induced Colitis
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hkchun@skku.edu
- 2Department of Surgery, University of Melbourne, Victoria, Australia.
- 3Center for Clinical Research, Samsung Biomedical Research Institute, Seoul, Korea.
- 4Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2212035
- DOI: http://doi.org/10.4174/jkss.2010.79.5.317
Abstract
- PURPOSE
Mesenchymal stem cells (MSCs) are surfacing as a new method of treatment for various diseases that have poor outcome with drug treatments. In this study, we investigated the effects of MSCs in a murine intestinal inflammation model mimicking human Crohn's disease (CD) using 2,4,5-trinitrobenzene sulfonic acid (TNBS).
METHODS
Colitis was induced by rectal administration of 2 mg of TNBS in 35% ethanol as experimental group compared to control group. Histological changes, surface molecules of T and B cells of the spleen and blood, and cytokine production (IFN-gamma, TNF-alpha, IL-4, IL-6, IL-10, and IL-12) were determined among 3 groups comprised of control group, TNBS group and TNBS/MSC group.
RESULTS
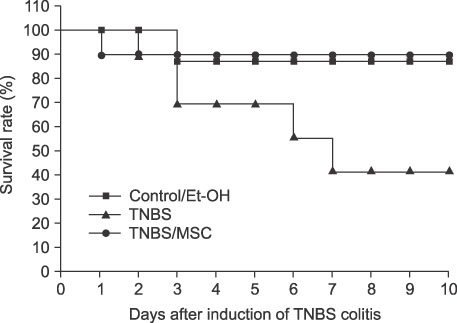
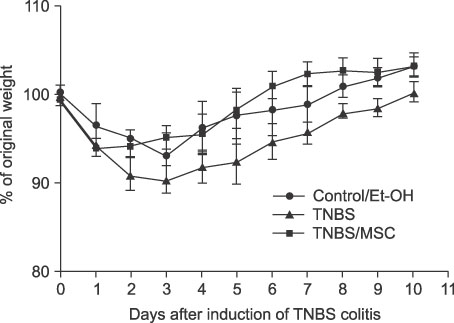
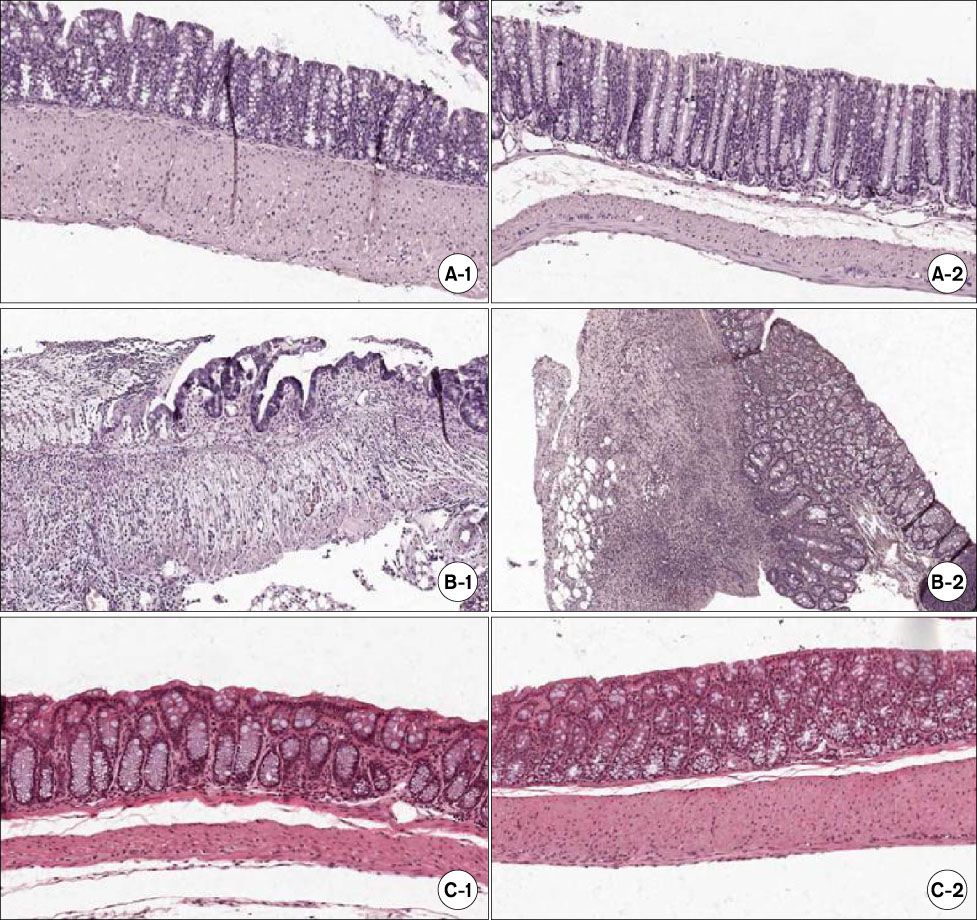
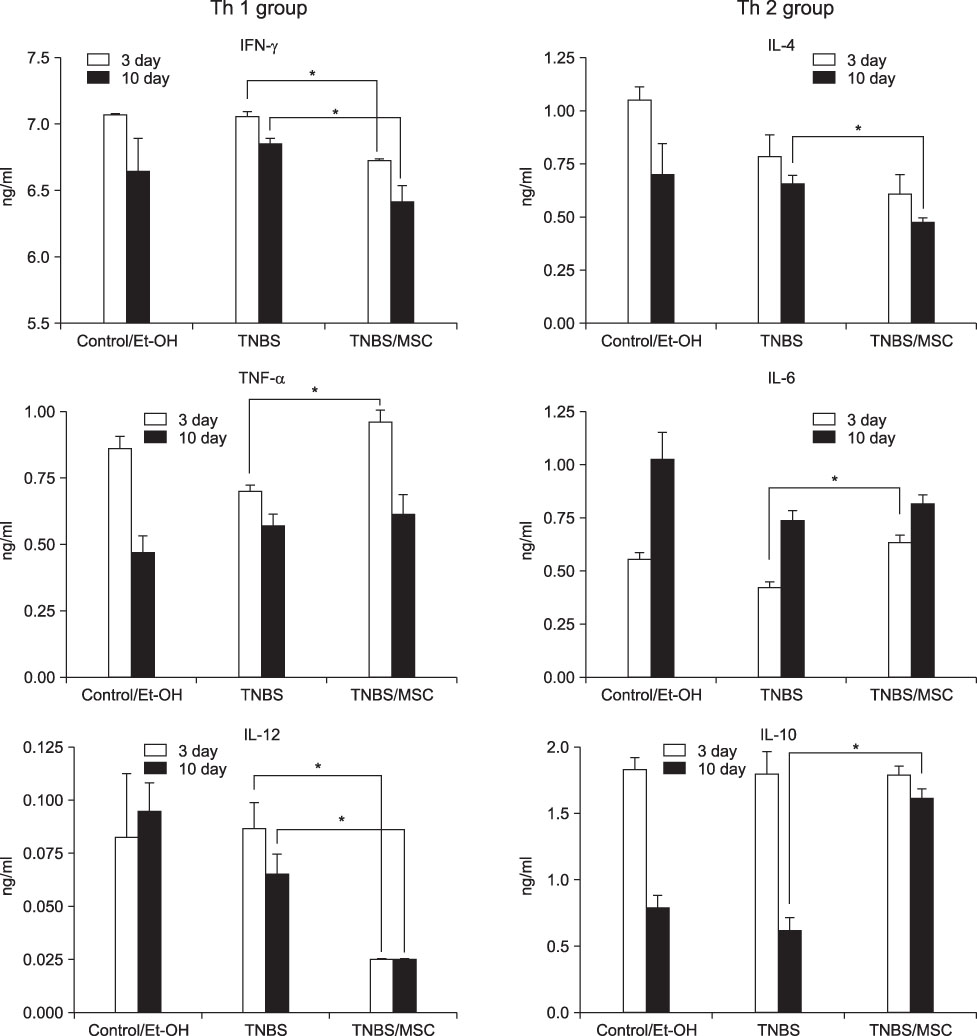
In the mice treated with MSCs, there was a decrease in the wasting disease process and inflammatory histopathological changes. There was also a decrease in pro-inflammatory T-helper 1 (Th1) cytokines IFN-gamma and IL-12 and T-helper 2 (Th2) cytokine IL-4. Anti-inflammatory cytokine IL-10 increased in mice treated with MSCs compared to colitic mice. The blood CD4+CD25+ T-regulatory cells also increased and splenic CD19 B-cells decreased.
CONCLUSION
The results of this study suggest that MSCs may have a therapeutic effect in controlling the Th1 and Th2 mediated immune response in patients with CD and aid in tissue regeneration.
MeSH Terms
-
Administration, Rectal
Animals
B-Lymphocytes
Colitis
Crohn Disease
Cytokines
Ethanol
Humans
Inflammation
Inflammatory Bowel Diseases
Interleukin-10
Interleukin-12
Interleukin-4
Interleukin-6
Mesenchymal Stromal Cells
Mice
Regeneration
Spleen
Tumor Necrosis Factor-alpha
Wasting Syndrome
Cytokines
Ethanol
Interleukin-10
Interleukin-12
Interleukin-4
Interleukin-6
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991. 325:928–937.2. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998. 115:182–205.3. MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000. 51:2–9.4. Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001. 120:622–635.5. Geboes K. From inflammation to lesion. Acta Gastroenterol Belg. 1994. 57:273–284.6. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003. 3:521–533.7. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003. 3:331–341.8. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007. 117:514–521.9. Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002. 122:1592–1608.10. Van Deventer SJ. Immunotherapy of Crohn's disease. Scand J Immunol. 2000. 51:18–22.11. Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007. 262:509–525.12. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.13. Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003. 10:228–241.14. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005. 105:1815–1822.15. Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004. 363:1411–1412.16. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–1441.17. Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, et al. Allogeneic bone marrow-derived flk-1+Sca-1-mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol. 2004. 32:861–867.18. Ertel W, Keel M, Neidhardt R, Steckholzer U, Kremer JP, Ungethuem U, et al. Inhibition of the defense system stimulating interleukin-12 interferon-gamma pathway during critical illness. Blood. 1997. 89:1612–1620.19. Miner KT, Croft M. Generation, persistence, and modulation of Th0 effector cells: role of autocrine IL-4 and IFN-gamma. J Immunol. 1998. 160:5280–5287.20. Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993. 362:245–248.21. Chen L, Grabowski KA, Xin JP, Coleman J, Huang Z, Espiritu B, et al. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol. 2004. 172:2059–2066.22. Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997. 89:4100–4103.23. Rattigan Y, Hsu JM, Mishra PJ, Glod J, Banerjee D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res. Epub 2010 Jul 13.24. Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci U S A. 2005. 102:17418–17423.25. Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004. 16:89–98.26. Veltkamp C, Sartor RB, Giese T, Autschbach F, Kaden I, Veltkamp R, et al. Regulatory CD4+CD25+ cells reverse imbalances in the T cell pool of bone marrow transplanted TGepsilon26 mice leading to the prevention of colitis. Gut. 2005. 54:207–214.27. Gärdby E, Chen XJ, Lycke NY. Impaired CD40-signalling in CD19-deficient mice selectively affects Th2-dependent isotype switching. Scand J Immunol. 2001. 53:13–23.28. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006. 8:Suppl 2. S3.29. Lee J, Kim MS, Kim EY, Park HJ, Chang CY, Jung DY, et al. 15-deoxyspergualin prevents mucosal injury by inhibiting production of TNF-alpha and down-regulating expression of MD-1 in a murine model of TNBS-induced colitis. Int Immunopharmacol. 2007. 7:1003–1012.30. Lee J, Lee EN, Kim EY, Park HJ, Chang CY, Jung DY, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol Lett. 2005. 101:210–216.31. García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell-based therapy. Int J Colorectal Dis. 2003. 18:451–454.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Local Mesenchymal Stem Cell Therapy in Experimentally Induced Colitis in the Rat
- The Therapeutic Efficacy of Tonsil-derived Mesenchymal Stem Cells in Dextran Sulfate Sodium-induced Acute Murine Colitis Model
- Changes of colonic endocrine cells in trinitrobenzene sulfonic acid (TNBS)-induced rat colitis
- Gingival Mesenchymal Stem Cells: A Periodontal Regenerative Substitute
- Saccharomyces boulardii Reduced Intestinal Inflammation in Mice Model of 2,4,6-trinitrobencene Sulfonic Acid Induced Colitis: Based on Microarray