J Korean Soc Endocrinol.
2006 Feb;21(1):32-39. 10.3803/jkes.2006.21.1.32.
Effect of High Concentration of Estradiol on Thyroid Specific Genes Expression and Cell Growth
- Affiliations
-
- 1Department of Internal Medicine, Dankook University, School of Medicine, Cheonam, Korea.
- KMID: 2200655
- DOI: http://doi.org/10.3803/jkes.2006.21.1.32
Abstract
-
BACKGROUND: Since various thyroid diseases have dominant prevalence in women, it has been suggested that female sex hormone have important role on thyroid cell physiology. Interestingly, many thyroid disorders are newly diagnosed or changed their course around the period of high estrogen status, such as pregnancy. In this study, we questioned whether high concentration of estrogen could modulate thyroid cell function.
METHODS
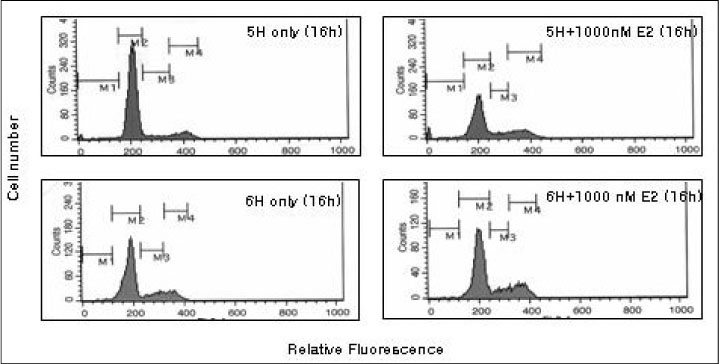
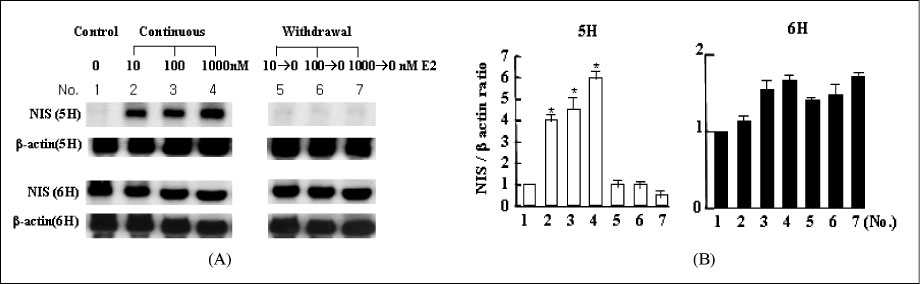
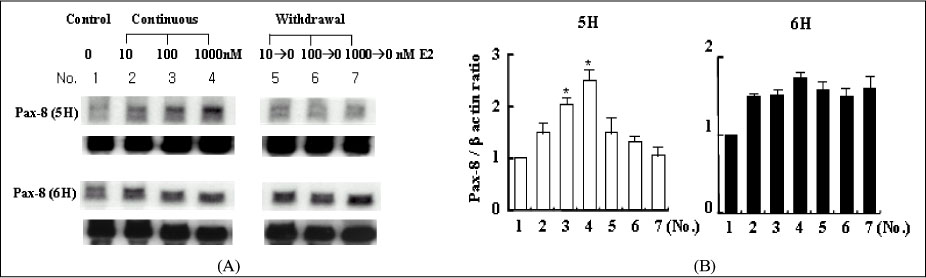
We treated normal rat thyroid FRTL-5 cell line with different time and concentration of estradiol. Using cell count, FACscan, and Northern blot analysis, we compared the changes of cell growth, cell cycle progression and thyroid specific genes expression. To evaluate the influence of thyroid stimulating hormone (TSH), all experiment was designed as two different sets, with (6H) or without TSH (5H).
RESULTS
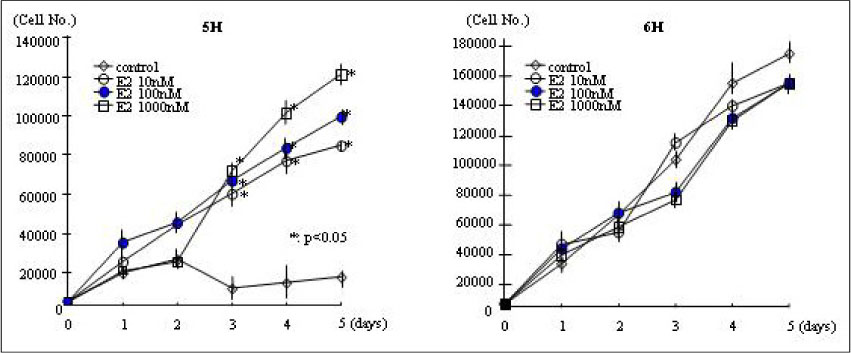
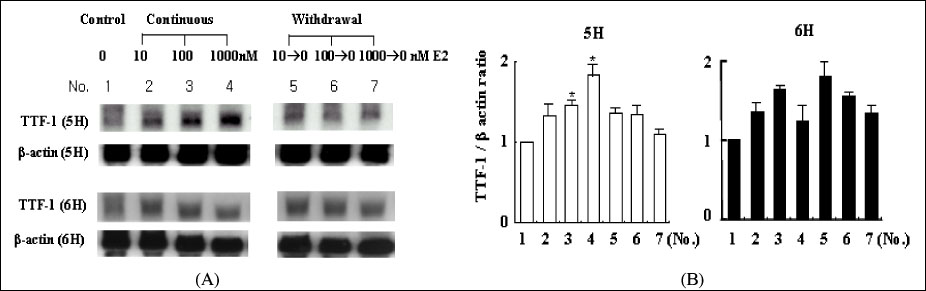
The concentration of 10-1000 nM estradiol had definite stimulatory function on thyroid cell growth in 5H condition as concentration dependent manner. FACscan revealed the increased cell growths were related to G1/S progression. The Pax-8, TTF-1 and NIS gene expressions were dramatically increased in 10-1000 nM of estradiol, too. With TSH (6H), however, we could not find any cell growth stimulating effects with 10-1000 nM of estradiol.
CONCLUSION
High concentration of estradiol is one of important control factor for thyroid growth and thyroid specific genes expression, especially in 5H condition. It indicate that exposure to high concentration of female sex hormone, such as pregnancy, can be a direct stimulating factor to various thyroid function and related to autoimmune or nodular thyroid diseases around the period of pregnancy.
MeSH Terms
Figure
Reference
-
1. Vanderpump M, Tunbridge W. Braverman L, Utiger R, editors. The epidemiology of thyroid diseases. The Thyroid: A fundamental and Clinical Text. 1996. 7th ed. Philadelphia: Lippincott Raven;474–482.2. Ishii K, Hayashi A, Tamaoka A, Mizusawa H, Shoji S. A case of Hashimoto's encephalopathy with a relapsing course related to menstrual cycle. Rinsho Shinkeigaku. 1993. 33:995–997.3. De Leo V, D'Antona D, Lanzetta D. Thyrotropin secretion before and after ovariectomy in premenopausal women. Gynecol Endocrinol. 1993. 7:279–283.4. Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002. 12:63–68.5. Watanabe H, Kawabe H. Relapse of Graves' disease after estrogen therapy for climacteric symptoms. Clin Endocrinol. 1996. 45:505–507.6. Ogard CG, Ogard C, Almdal TP. Thyroid-associated orbitopathy developed during hormone replacement therapy. Acta Ophthalmol Scan. 2001. 79:426–427.7. Irizarry S, Paniagua M, Pincus G, Janer JL, Frias Z. Effect of cyclic administration of certain progestin-estrogen combination on the 24-hour raioiodine thyroid uptake. J Clin Endocrinol Metab. 1966. 26:6–10.8. D'Angelo SA, Fisher JS. Influence of estrogen on the pituitary-thyroid system of the female rat: mechanism and loci of action. Endocrinology. 1969. 84:117–122.9. Hiasa Y, Nishioka H, Kitahori Y, Yane K, Nakaoka S, Ohshima M, Konishi N, Nishii K, Kitamura M, Matsunga T. Immunohistochemical analysis of estrogen and progesteron receptor in 313 paraffin section cases of human thyroid tissue. Oncology. 1993. 50:132–136.10. Van Hoeven K, Menedez-Botet C, Strong E, Huvos A. Estrogen and progesteron receptor content in human thyroid diseases. Am J Clin Pathol. 1993. 99:175–181.11. Valle LD, Ramina A, Vianello S, Fassina A, Paola B, Colombo L. Potential for estrogen synthesis and action in human normal and neoplastic thyroid tissue. J Clin Endocrinol Metab. 1998. 83:3702–3709.12. Furlanetto TW, Nguyen LQ, Jameson JL. Estradiol increases proliferation and down regulates the sodium/iodide symporter gene in FRTL-5 cell. Endocrinology. 1999. 140:5705–5711.13. Manole D, Schilknecht N, Gosnell B, Anams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanism. J Clin Endocrinol Metab. 2001. 86:1072–1077.14. Struve CW, Haupt S, Ohlen S. Influence of frequency of previous pregnancy on the prevalence of thyroid nodules in women without clinical evidence of thyroid disease. Thyroid. 1993. 3:7–9.15. Kung AWC, Chau MT, Lao TT, Tam SCF, Low LCK. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab. 2002. 87:1010–1014.16. Smallridge RC. Postpartum thyroid diseases through the ages: a historical view. Thyroid. 1999. 9:671–673.17. Speroff L, Glass RH, Kase NG. The endocrinology of pregnancy. Clinical gynecologic endocrinology and infertility. 1999. 6th ed. Maryland, Lippincott: Williams & Wilkins;276–335.18. Mendelsohn M, Karas R. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999. 340:1801–1811.19. Smyth PPA. Variation in iodine handling during normal pregnancy. Thyroid. 1999. 9:637–642.20. Glinoer D. What happens to the normal thyroid during pregnancy? Thyroid. 1999. 9:631–635.21. Glinoer D. The regulation of thyroid functions in pregnancy: pathway of endocrine adaptation from physiology to pathology. Endocr Rev. 1997. 18:404–433.22. Davies TF. The thyroid immunology of the postpartum period. Thyroid. 1999. 9:675–684.23. Buchsbaum DJ, Chaudhuri TR, Zinn KR. Radiotarget gene therapy. Nucl Med. 2005. 46:179–186.24. Damante G, Lauro RD. Thyroid-specific gene expression. Biochim Biophys Acta. 1994. 1218:225–266.25. Plachov DK, Chowdhury C, Walther D, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990. 110:643–651.26. Ohno M, Zannini M, Levy O, Carrasco N, Lauro RD. The paired-domain transcription factor Pax-8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclin-AMP-Dependent transcription. Mol Cell Biol. 1999. 19:2051–2060.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of estrogen on growth hormone receptor expression of human periodontal ligament cell line
- Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells
- The Effect of 17beta-Estradiol on the Gene Expression of IGF-I and Bone Matrix Protein in the Osteoblast-Like Cell
- Effects of sex hormone on secretion of growth hormone
- Correlations amongst Insulin-like Growth Factor-I, Insulin-like Growth Factor Binding Protein-3, Growth Hormone and Estradiol in Follicular Fluid of the Patients with Ovulation Induction