J Korean Soc Endocrinol.
2005 Jun;20(3):230-241. 10.3803/jkes.2005.20.3.230.
Mechanism of Castration-induced Apoptosis of Ventral Prostate in Rat
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, Chungnam National University, Daejeon, Korea.
- 2Department of Physiology, College of Medicine, Chungnam National University, Daejeon, Korea.
- 3Research Institute for Medical Science, Chungnam National University, Daejeon, Korea.
- 4Cancer Research Institute, Chungnam National University, Daejeon, Korea.
- KMID: 2200563
- DOI: http://doi.org/10.3803/jkes.2005.20.3.230
Abstract
-
BACKGROUNDS: Castration-induced androgen deprivation triggers a sequence of events, which activates apoptotic cell death of the androgen-dependent epithelial cells within the rat ventral prostate. To investigate the mechanism of castration-dependent apoptosis in the rat ventral prostate, the regulation of apoptosis-related genes was been investigated.
METHODS
Azaline B was subcutaneously injected into Sprague-Dawley rat. The Fas receptor (Fas), Fas ligand (FasL) and bcl-2 mRNA, as well as the protein levels were detected by RT-PCR and Western blot analyses. Azaline B-dependent apoptosis was determined using TUNEL and a DNA fragmentation assay. The transacting factor of the FasL promoter was identified by DNA footprinting and a DNA mobility shift assay.
RESULTS
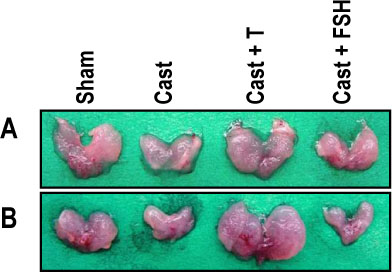
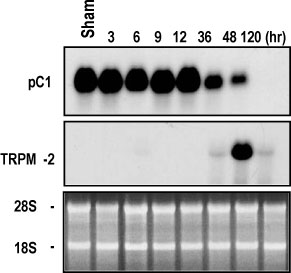
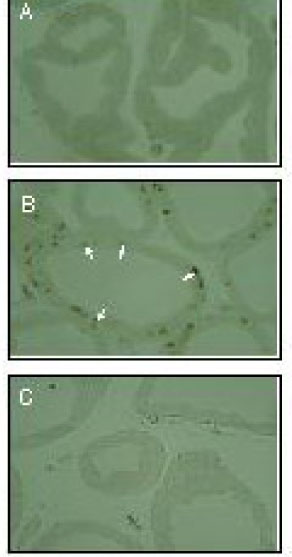
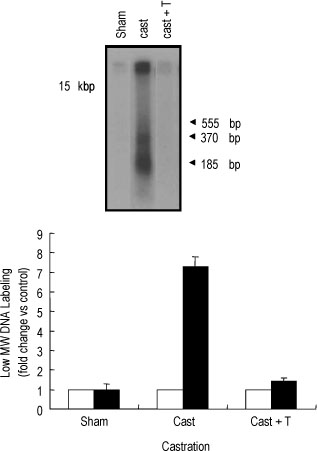
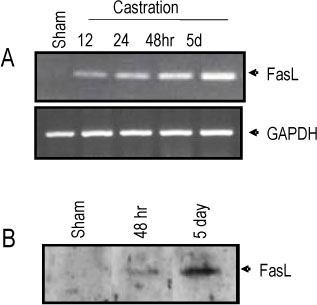
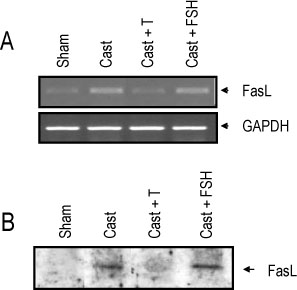
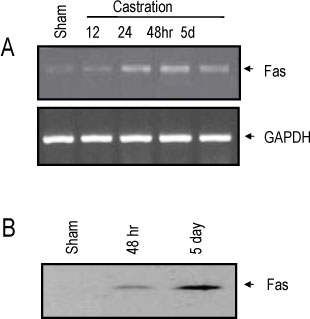
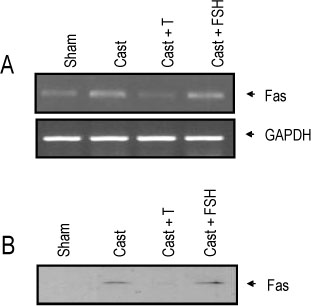
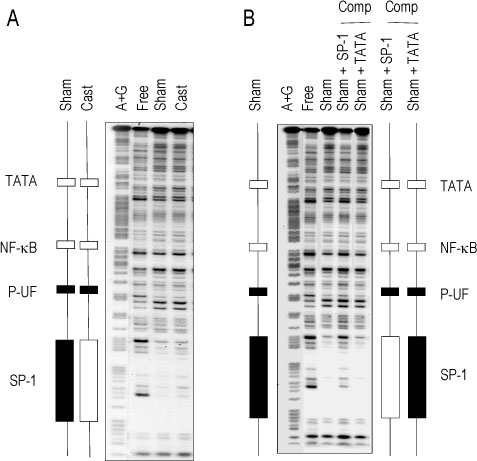
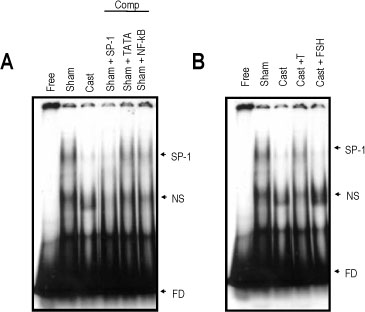
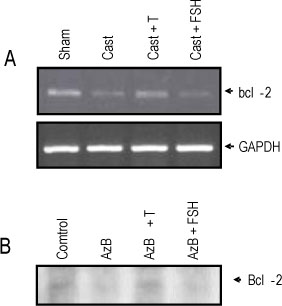
The rat prostate was regressed after castration, with and the involuted ventral prostate regenerated by testosterone pretreatment, but not by that with FSH. Apoptosis of the ventral prostate was detected, after castration, using toluidine blue staining, a TUNEL assay and an apoptotic DNA fragmentation assay. The levels of Fas, FasL mRNA and protein were increased after castration. In the DNase I footprinting assay, using the FasL promoter and a nuclear extract prepared from a control prostate, at least two sites were protected: the SP-1 binding site at -283 bp and the prostate-unidentified factor(P-UF) binding site at -247 bp. The SP-1 binding activity vanished in the nuclear extract prepared from castrated rats. In the DNA mobility shift assay, the SP-1 binding activity was slightly decreased after castration. Both the Bcl-2 mRNA and Bcl-2 protein were downregulated after castration.
CONCLUSION
These results suggest that the Fas/FasL system and Bcl-2 may be important to castrationdependent apoptosis in the rat ventral prostate, with SP-1 related to the castration-dependent regulation of the FasL gene
MeSH Terms
-
Animals
Antigens, CD95
Apoptosis*
Binding Sites
Blotting, Western
Castration
Cell Death
Deoxyribonuclease I
DNA
DNA Footprinting
DNA Fragmentation
Electrophoretic Mobility Shift Assay
Epithelial Cells
Fas Ligand Protein
In Situ Nick-End Labeling
Prostate*
Rats*
Rats, Sprague-Dawley
RNA, Messenger
Testosterone
Tolonium Chloride
Antigens, CD95
DNA
Deoxyribonuclease I
Fas Ligand Protein
RNA, Messenger
Testosterone
Tolonium Chloride
Figure
Reference
-
1. Smith CA, Williams GT, Kingstone R, Jenkinson EJ, Owen JJT. Antibodies to CD3/T-cell receptor complex induce cell death by apoptosis in immature T cells in thymic cultures. Nature. 1995. 374:811–814.2. Kerr JFR, Searle J, Harmon BV, Bishop CJ. Potten CS, editor. Apoptosis. Perspectives on Mammalian cell Death. 1987. Oxford University Press;93–94.3. Tsujimoto Y, Shimizu S, Eguchi Y, Kamiike W, Matsuda H. Bcl-2 and Bcl- XL block apoptosis as well as necrosis: possible involvement of common mediators in aopototic and necrotic signa transduction pathway. Leukemia. 1997. 11:380–382.4. Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, Li Weber M, Richards S, Dhein J, Trauth BC, Pansting IK, Krammer PH. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992. 267:10709–10715.5. Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993. 75:1169–1178.6. Nagata S, Golstein P. The Fas Death Factor. Science. 1995. 267:1449–1456.7. Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994. 1:365–371.8. Tanaka M, Itai T, Adachi M, Nagata S. Down regulation of Fas ligand by shedding. Nat Med. 1998. 4:31–36.9. Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond AH, Nagata S. Fas ligand in human serum. Nat Med. 1996. 2:317–322.10. Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995. 14:1129–1135.11. Laouar Y, Sarukhan A, Pasqualetto V, Garcia C, Ezine S. Involvement of the Fas (CD95) system in peripheral cell death and lympoid organ development. Eur J Immunol. 1998. 28:1078–1088.12. Shiraki K, Jsuji N, Shioda T, Isselbacher KJ, Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA. 1997. 94:6420–6425.13. Suzuki A, Matsuzawa A, Iguchi T. Down regulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tract. Oncogene. 1996. 13:31–37.14. Isaacs JT. Antagonistic effect of androgens on prostatic cell death. Prostate. 1984. 5:545–557.15. Chung WLK, McFadden DK. Sex steroid imprinting and prostate growth. Invest Urol. 1980. 17:337–342.16. English HF, Kypianou N, Isaacs JT. Relationship between DNA fragmentation and apoptosis in the programmed cell death in the rat prostate following castration. The Prostate. 1989. 15:233–250.17. Kyprianou N, English HF, Isaacs JT. Activation of a Ca2+-Mg2+-dependent endonuclease as an early event in castration induced prostatic cell death. Prostate. 1988. 13:103–117.18. Lim K, Park C, Kim YK, Yun KA, Son MY, Lee YC, Park JI, Lee JH, Sul CK, Lee CS, Park SK, Hwang BD. Association of castration-dependent early induction of c-myc expression with a cell proliferation of the ventral prostate gland in rat. Exp Mol Med. 2000. 32:216–221.19. Nickerson T, Pollak M, Huynh H. Castration- induced apoptosis in the rat ventral prostate is associated with increased expression of genes encoding insulin-like growth factor binding proteins 2, 3, 4 and 5. Endocrinology. 1998. 139:807–810.20. Suzuki A, Masao E, Eguchi Y, Matsuzawa A, Nagata S, Tsujimoto Y, Iguchi T. Involvement of Fas in regression of vaginal epithelia after ovariectomy and during an estrous cycle. EMBO J. 1996. 15:211–215.21. Taille de la A, Chen MW, Shabsign A, Bagiella E, Kiss A, Buttyan R. Fas antigen/CD-95 upregulation and activation during castration- induced regression of the rat ventral prostate gland. The Prostate. 1999. 40:89–96.22. Tilly JL, Hsueh AJM. Microscale autoradiographic method for qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol. 1993. 154:519–526.23. Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997. 138:2081–2088.25. Virca GD, Northemann W, Shiels BR, Widera G, Broome S. Simplified northern blot hybridization using 5% sodium dodecyl sulfate. BioTechniques. 1990. 8:370–371.26. Lim K, Yoo JH, Kim KY, Kweon GR, Kwak ST, Hwang BD. Testosterone regulation of proto-oncogene c-myc expression in primary Sertoli cell cultures from prepubertal rats. J Androl. 1994. 15:543–550.27. Gorski K, Carneiro M, Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986. 47:767–776.28. Lim K, Chae CB. Presence of receptor protein for testis-specific H2B (TH2B) histone gene in early stages of spermatogenesis. J Biol Chem. 1992. 267:15271–15273.29. Kalb VF Jr, Bernlohr RW. A new spectrophtomeric assay for protein in cell extracts. Anal Biochem. 1977. 82:362–371.30. Parker MG, White R, Williams JG. Cloning and characterization of androgen- dependent mRNA from rat ventral prostate. J Biol Chem. 1980. 255:6996–7001.31. Montpetit ML, Lawless KR, Tenniswood M. Androgen repressed messages in the rat ventral prostate. Prostate. 1986. 8:25–36.32. Schulze-osthoff K, Ferrari D, Los M, Wesselborg S, Marcus EP. Apoptosis signaling by death receptors. Eur J Biochem. 1998. 254:439–459.33. Holz-Heppelmann CJ, Algreciras A, Badley AD, Paya CV. Transcriptional regulation of the human FasL promoter-enhancer region. J Biol Chem. 1998. 273:4416–4423.34. McClure RF, Heppelmann CJ, Paya CV. Constructive Fas ligand gene transcription in Sertoli cells is regulated by Sp1. J Biol Chem. 1999. 274:7756–7762.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of A Potent Calcium Channel Blocker, Nifedipine, on the Castration-induced Apoptosis of the Rat Ventral Prostate
- Association of castration-dependent early induction of c-myc expression with a cell proliferation of the ventral prostate gland in rat
- The Effect of Testosterone on the Rat Penis and Accessory Sex Glands Following Castration
- Morphological and Quantitative Analysis on the Expression of Bax in the Rat Prostate following Castration
- Effects of Finasteride and Castration on Rat Ventral Prostate