J Korean Neurosurg Soc.
2016 Mar;59(2):106-116. 10.3340/jkns.2016.59.2.106.
Bacitracin Inhibits the Migration of U87-MG Glioma Cells via Interferences of the Integrin Outside-in Signaling Pathway
- Affiliations
-
- 1Brain Tumor Research Laboratory and Department of Neurosurgery Chonnam National University Research Institute of Medical Sciences, Chonnam National University Hwasun Hopital and Medical School, Hwasun, Korea. sjung@jnu.ac.kr
- KMID: 2192039
- DOI: http://doi.org/10.3340/jkns.2016.59.2.106
Abstract
OBJECTIVE
Protein disulfide isomerase (PDI) acts as a chaperone on the cell surface, and it has been reported that PDI is associated with the tumor cell migration and invasion. The aims of this study are to investigate the anti-migration effect of bacitracin, which is an inhibitor of PDI, and the associated factor in this process.
METHODS
U87-MG glioma cells were treated with bacitracin in 1.25, 2.5, 3.75, and 5.0 mM concentrations. Western blot with caspase-3 was applied to evaluate the cytotoxicity of bacitracin. Adhesion, morphology, migration assays, and organotypic brain-slice culture were performed to evaluate the effect of bacitracin to the tumor cell. Western blot, PCR, and gelatin zymography were performed to investigate the associated factors. Thirty glioma tissues were collected following immunohistochemistry and Western blot.
RESULTS
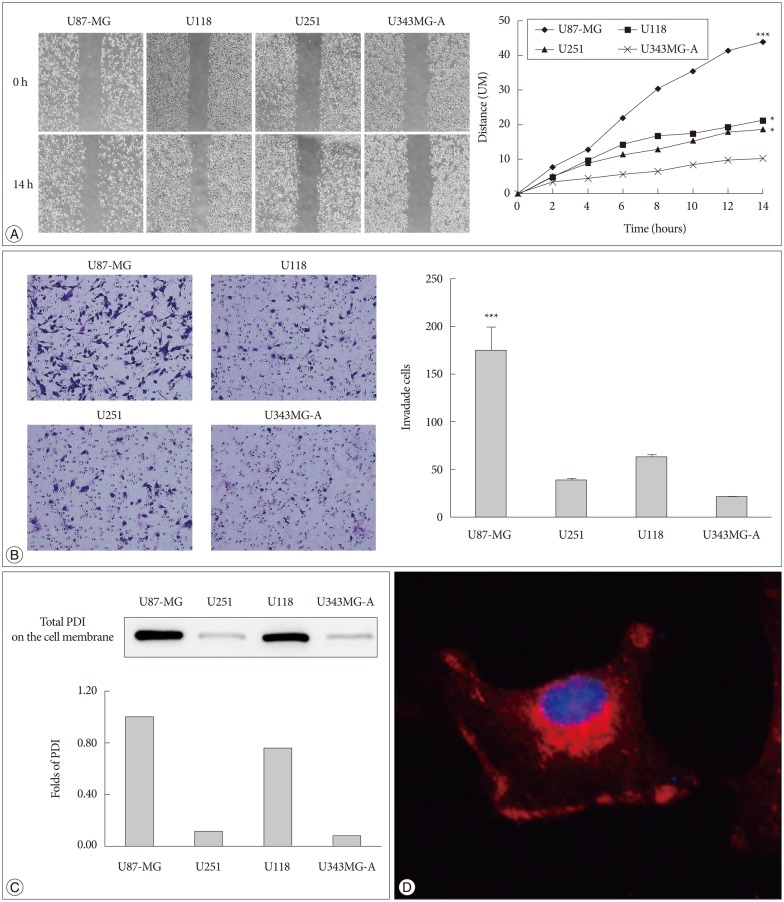
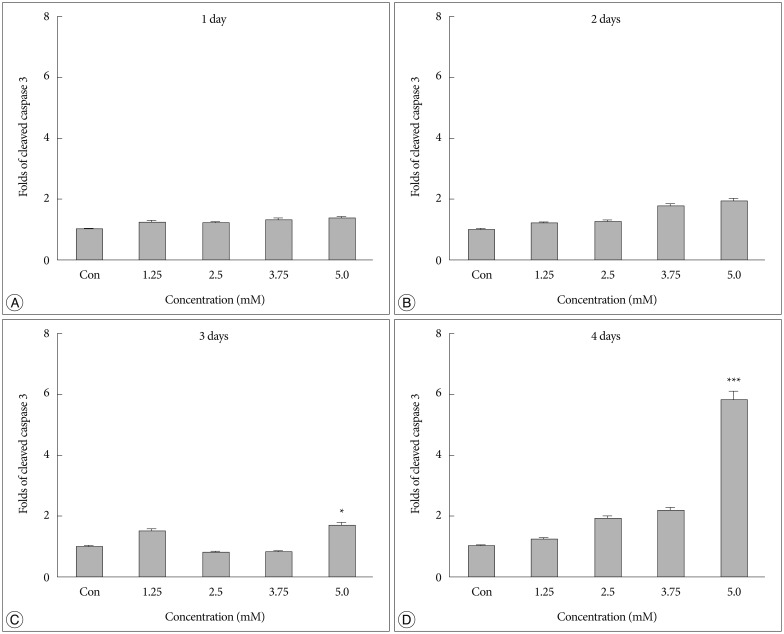
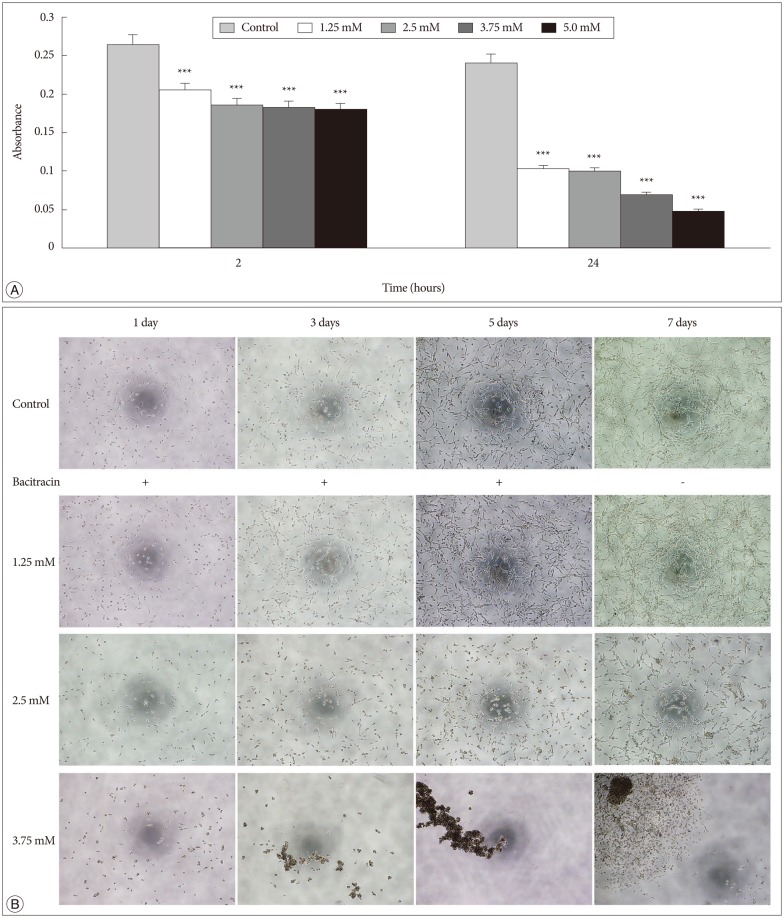
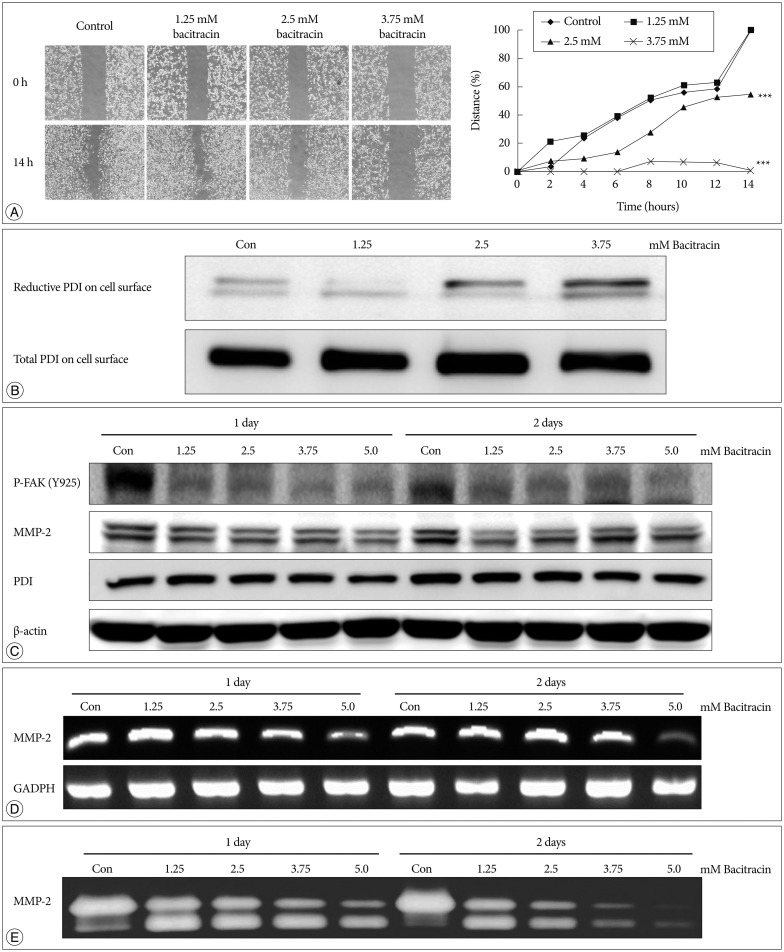
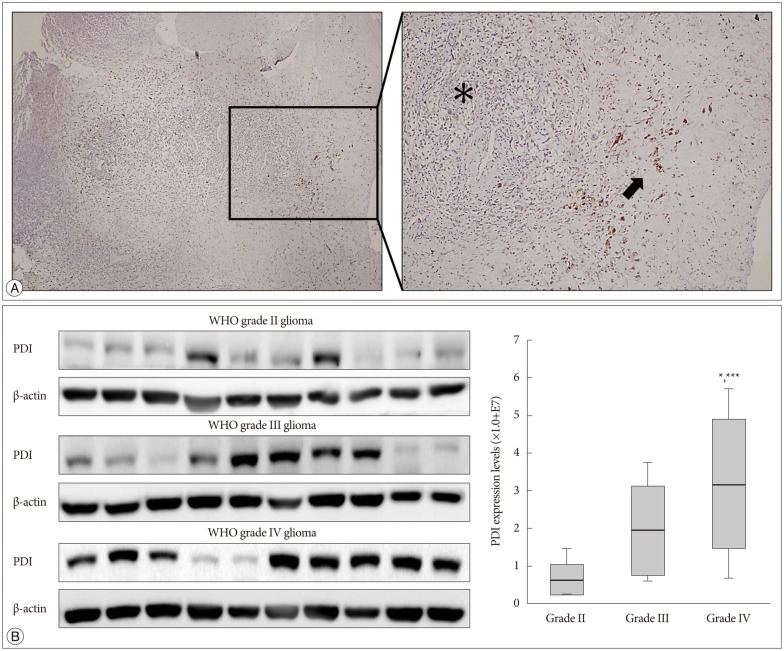
Bacitracin showed a cytotoxicity in 3rd (p<0.05) and 4th (p<0.001) days, in 5.0 Mm concentration. The cell adhesion significantly decreased and the cells became a round shape after treated with bacitracin. The migration ability, the expression of phosphorylated focal adhesion kinase (p-FAK) and matrix metalloproteinase-2 (MMP-2) decreased in a bacitracin dose- and time-dependent manner. The U87-MG cells exhibited low-invasiveness in the 2.5 mM, compared with the untreated in organotypic brain-slice culture. PDI was expressed in the tumor margin, and significantly increased with histological glioma grades (p<0.001).
CONCLUSION
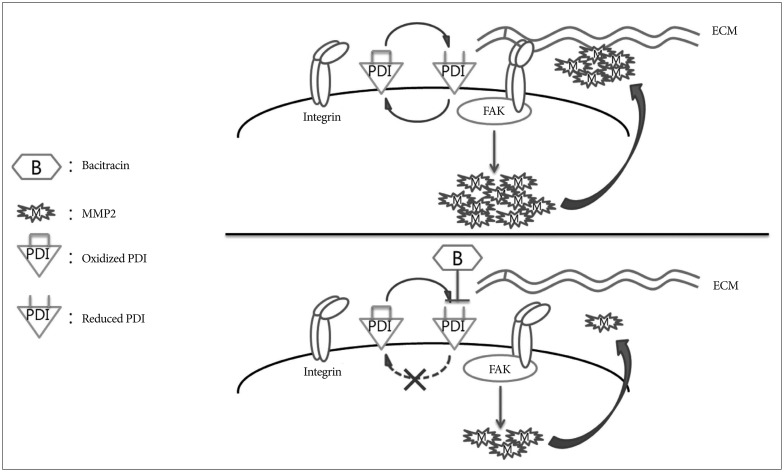
Bacitracin, as a functional inhibitor of PDI, decreased the phosphorylated FAK and the secreted MMP-2, which are the downstream of integrin and play a major role in cell migration and invasion, might become one of the feasible therapeutic strategies for glioblastoma.
Keyword
MeSH Terms
-
Bacitracin*
Blotting, Western
Caspase 3
Cell Adhesion
Cell Movement
Focal Adhesion Protein-Tyrosine Kinases
Gelatin
Glioblastoma
Glioma*
Immunohistochemistry
Matrix Metalloproteinase 2
Polymerase Chain Reaction
Protein Disulfide-Isomerases
Bacitracin
Caspase 3
Focal Adhesion Protein-Tyrosine Kinases
Gelatin
Matrix Metalloproteinase 2
Protein Disulfide-Isomerases
Figure
Reference
-
1. Benham AM. The protein disulfide isomerase family : key players in health and disease. Antioxid Redox Signal. 2012; 16:781–789. PMID: 22142258.
Article2. Deep G, Kumar R, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits fibronectin induced motility, invasiveness and survival in human prostate carcinoma PC3 cells via targeting integrin signaling. Mutat Res. 2014; 768:35–46. PMID: 25285031.
Article3. Di Santo N, Ehrisman J. Research perspective : potential role of nitazoxanide in ovarian cancer treatment. Old drug, new purpose? Cancers (Basel). 2013; 5:1163–1176. PMID: 24202339.
Article4. Dickerhof N, Kleffmann T, Jack R, McCormick S. Bacitracin inhibits the reductive activity of protein disulfide isomerase by disulfide bond formation with free cysteines in the substrate-binding domain. FEBS J. 2011; 278:2034–2043. PMID: 21481187.
Article5. Essex DW. The role of thiols and disulfides in platelet function. Antioxid Redox Signal. 2004; 6:736–746. PMID: 15242555.
Article6. Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995; 86:2168–2173. PMID: 7662965.
Article7. Goplen D, Wang J, Enger PØ, Tysnes BB, Terzis AJ, Laerum OD, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006; 66:9895–9902. PMID: 17047051.
Article8. Hatahet F, Ruddock LW. Substrate recognition by the protein disulfide isomerases. FEBS J. 2007; 274:5223–5234. PMID: 17892489.
Article9. Huang G, Ho B, Conroy J, Liu S, Qiang H, Golubovskaya V. The microarray gene profiling analysis of glioblastoma cancer cells reveals genes affected by FAK inhibitor Y15 and combination of Y15 and temozolomide. Anticancer Agents Med Chem. 2014; 14:9–17. PMID: 23387973.
Article10. Janouskova H, Maglott A, Leger DY, Bossert C, Noulet F, Guerin E, et al. Integrin α5β1 plays a critical role in resistance to temozolomide by interfering with the p53 pathway in high-grade glioma. Cancer Res. 2012; 72:3463–3470. PMID: 22593187.
Article11. Jiang XM, Fitzgerald M, Grant CM, Hogg PJ. Redox control of exofacial protein thiols/disulfides by protein disulfide isomerase. J Biol Chem. 1999; 274:2416–2423. PMID: 9891011.
Article12. Johnson GG, White MC, Grimaldi M. Stressed to death : targeting endoplasmic reticulum stress response induced apoptosis in gliomas. Curr Pharm Des. 2011; 17:284–292. PMID: 21348829.13. Johnson GG, White MC, Wu JH, Vallejo M, Grimaldi M. The deadly connection between endoplasmic reticulum, Ca2+, protein synthesis, and the endoplasmic reticulum stress response in malignant glioma cells. Neuro Oncol. 2014; 16:1086–1099. PMID: 24569545.
Article14. Jung S, Kim HW, Lee JH, Kang SS, Rhu HH, Jeong YI, et al. Brain tumor invasion model system using organotypic brain-slice culture as an alternative to in vivo model. J Cancer Res Clin Oncol. 2002; 128:469–476. PMID: 12242510.
Article15. Jung TY, Jung S, Ryu HH, Jeong YI, Jin YH, Jin SG, et al. Role of galectin-1 in migration and invasion of human glioblastoma multiforme cell lines. J Neurosurg. 2008; 109:273–284. PMID: 18671640.
Article16. Karala AR, Ruddock LW. Bacitracin is not a specific inhibitor of protein disulfide isomerase. FEBS J. 2010; 277:2454–2462. PMID: 20477872.
Article17. Kim CS, Jung S, Jung TY, Jang WY, Sun HS, Ryu HH. Characterization of invading glioma cells using molecular analysis of leading-edge tissue. J Korean Neurosurg Soc. 2011; 50:157–165. PMID: 22102942.
Article18. Lahav J, Gofer-Dadosh N, Luboshitz J, Hess O, Shaklai M. Protein disulfide isomerase mediates integrin-dependent adhesion. FEBS Lett. 2000; 475:89–92. PMID: 10858494.
Article19. Lahav J, Wijnen EM, Hess O, Hamaia SW, Griffiths D, Makris M, et al. Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin alpha2beta1. Blood. 2003; 102:2085–2092. PMID: 12791669.
Article20. Li X, Ishihara S, Yasuda M, Nishioka T, Mizutani T, Ishikawa M, et al. Lung cancer cells that survive ionizing radiation show increased integrin α2β1- and EGFR-dependent invasiveness. PLoS One. 2013; 8:e70905. PMID: 23951036.
Article21. Liddington RC, Ginsberg MH. Integrin activation takes shape. J Cell Biol. 2002; 158:833–839. PMID: 12213832.
Article22. Lovat PE, Corazzari M, Armstrong JL, Martin S, Pagliarini V, Hill D, et al. Increasing melanoma cell death using inhibitors of protein disulfide isomerases to abrogate survival responses to endoplasmic reticulum stress. Cancer Res. 2008; 68:5363–5369. PMID: 18593938.
Article23. Ma J, Cui W, He SM, Duan YH, Heng LJ, Wang L, et al. Human U87 astrocytoma cell invasion induced by interaction of βig-h3 with integrin α5β1 involves calpain-2. PLoS One. 2012; 7:e37297. PMID: 22629380.
Article24. Mandel R, Ryser HJ, Ghani F, Wu M, Peak D. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Proc Natl Acad Sci U S A. 1993; 90:4112–4116. PMID: 8387210.
Article25. Ryu HH, Jung S, Jung TY, Moon KS, Kim IY, Jeong YI, et al. Role of metallothionein 1E in the migration and invasion of human glioma cell lines. Int J Oncol. 2012; 41:1305–1313. PMID: 22843066.
Article26. Shi Q, Hjelmeland AB, Keir ST, Song L, Wickman S, Jackson D, et al. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog. 2007; 46:488–496. PMID: 17219439.
Article27. Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003; 278:7607–7616. PMID: 12493773.
Article28. Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee NP, et al. Inhibition of prolyl 4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide resistance in malignant glioma via the endoplasmic reticulum stress response (ERSR) pathways. Neuro Oncol. 2013; 15:562–577. PMID: 23444257.
Article29. Terada K, Manchikalapudi P, Noiva R, Jauregui HO, Stockert RJ, Schilsky ML. Secretion, surface localization, turnover, and steady state expression of protein disulfide isomerase in rat hepatocytes. J Biol Chem. 1995; 270:20410–20416. PMID: 7657616.
Article30. Tian F, Zhou X, Wikström J, Karlsson H, Sjöland H, Gan LM, et al. Protein disulfide isomerase increases in myocardial endothelial cells in mice exposed to chronic hypoxia : a stimulatory role in angiogenesis. Am J Physiol Heart Circ Physiol. 2009; 297:H1078–H1086. PMID: 19617410.31. Tysnes BB, Mahesparan R. Biological mechanisms of glioma invasion and potential therapeutic targets. J Neurooncol. 2001; 53:129–147. PMID: 11716066.32. Wang EJ, Snyder RD, Fielden MR, Smith RJ, Gu YZ. Validation of putative genomic biomarkers of nephrotoxicity in rats. Toxicology. 2008; 246:91–100. PMID: 18289764.
Article33. Weston BS, Wahab NA, Roberts T, Mason RM. Bacitracin inhibits fibronectin matrix assembly by mesangial cells in high glucose. Kidney Int. 2001; 60:1756–1764. PMID: 11703593.
Article34. Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells : implications for radiotherapy of human glioblastoma. Cancer Res. 2001; 61:2744–2750. PMID: 11289157.35. Xu S, Sankar S, Neamati N. Protein disulfide isomerase : a promising target for cancer therapy. Drug Discov Today. 2014; 19:222–240. PMID: 24184531.36. Yu SJ, Yoon JH, Yang JI, Cho EJ, Kwak MS, Jang ES, et al. Enhancement of hexokinase II inhibitor-induced apoptosis in hepatocellular carcinoma cells via augmenting ER stress and anti-angiogenesis by protein disulfide isomerase inhibition. J Bioenerg Biomembr. 2012; 44:101–115. PMID: 22350012.
Article37. Zhang K, Ding W, Sun W, Sun XJ, Xie YZ, Zhao CQ, et al. Beta1 integrin inhibits apoptosis induced by cyclic stretch in annulus fibrosus cells via ERK1/2 MAPK pathway. Apoptosis. 2015; [Epub ahead of print].
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of chromosomal region maintenance 1 suppresses the migration and invasion of glioma cells via inactivation of the STAT3/MMP2 signaling pathway
- The Dose Dependent Effects of Ruxolitinib on the Invasion and Tumorigenesis in Gliomas Cells via Inhibition of Interferon Gamma-Depended JAK/STAT Signaling Pathway
- in vitro Biological Response of Malignant Glioma Cell Lines to Gamma Knife Irradiation
- Targeting Orthotopic Glioma in Mice with Genetically Engineered Salmonella typhimurium
- Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells