J Korean Neurosurg Soc.
2014 Mar;55(3):125-130. 10.3340/jkns.2014.55.3.125.
The Neuroprotective Effects of Carnosine in Early Stage of Focal Ischemia Rodent Model
- Affiliations
-
- 1Department of Neurosurgery, Seoul Medical Center, Seoul, Korea.
- 2Research Institute, Seoul Medical Center, Seoul, Korea. dohheekim@gmail.com
- KMID: 2191076
- DOI: http://doi.org/10.3340/jkns.2014.55.3.125
Abstract
OBJECTIVE
This study was conducted to elucidate neuroprotective effect of carnosine in early stage of stroke.
METHODS
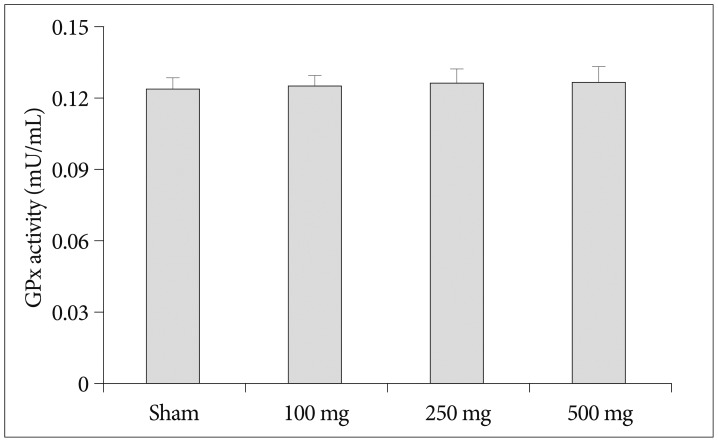
Early stage of rodent stroke model and neuroblastoma chemical hypoxia model was established by middle cerebral artery occlusion and antimycin A. Neuroprotective effect of carnosine was investigated with 100, 250, and 500 mg of carnosine treatment. And antioxidant expression was analyzed by enzyme linked immunosorbent assay (ELISA) and western blot in brain and blood.
RESULTS
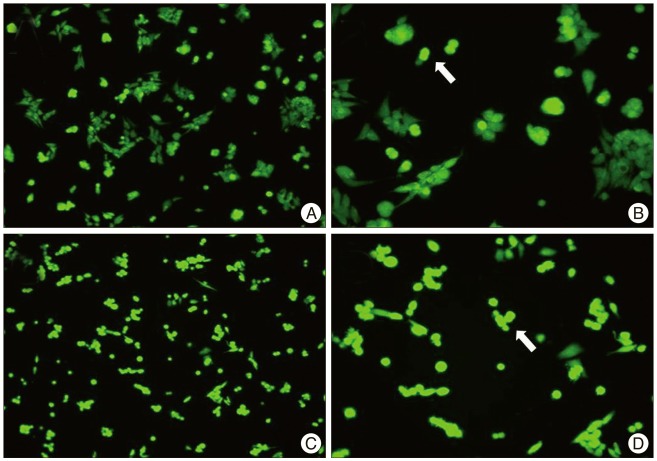
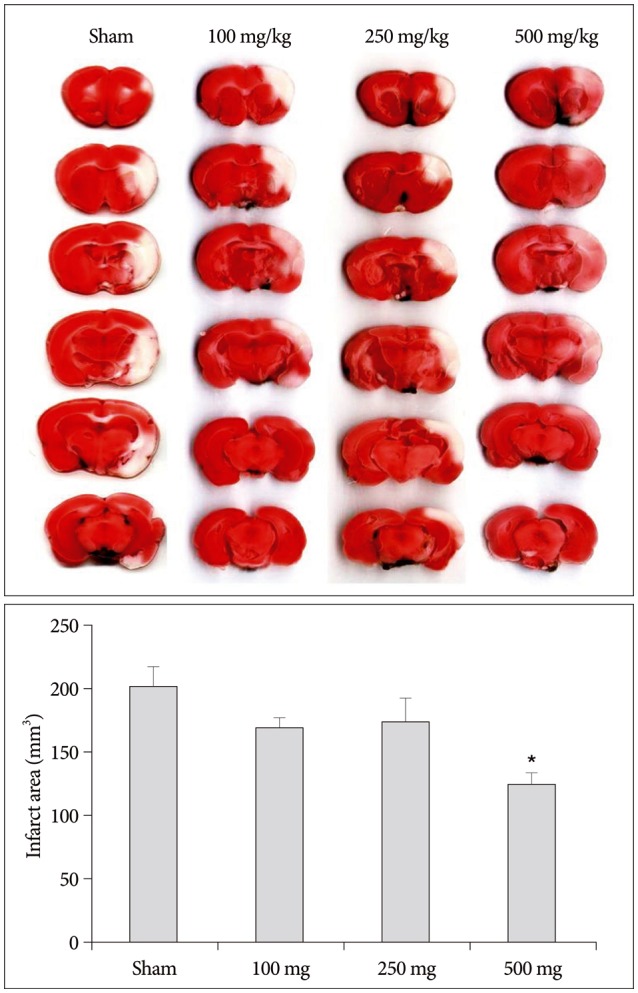
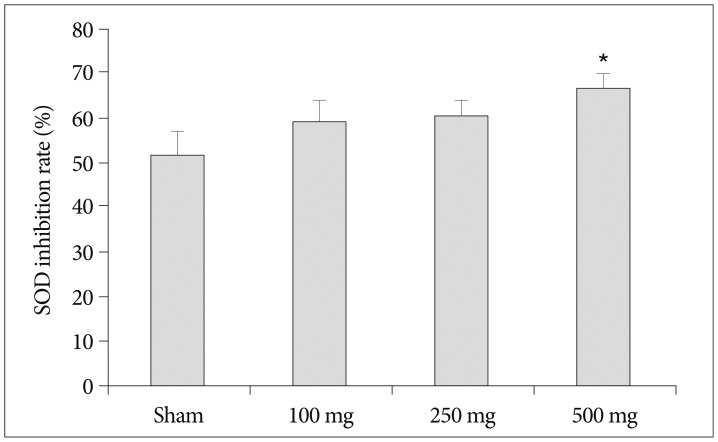
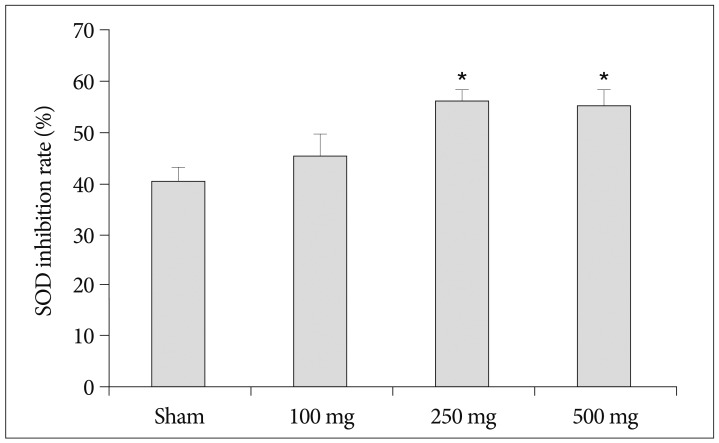
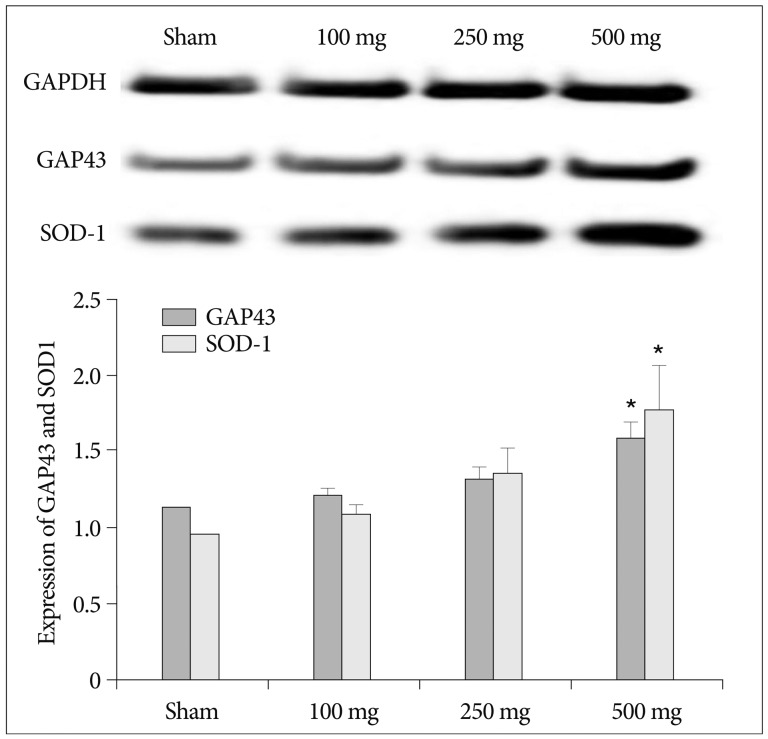
Intraperitoneal injection of 500 mg carnosine induced significant decrease of infarct volume and expansion of penumbra (p<0.05). The expression of superoxide dismutase (SOD) showed significant increase than in saline group in blood and brain (p<0.05). In the analysis of chemical hypoxia, carnosine induced increase of neuronal cell viability and decrease of reactive oxygen species (ROS) production.
CONCLUSION
Carnosine has neuroprotective property which was related to antioxidant capacity in early stage of stroke. And, the oxidative stress should be considered one of major factor in early ischemic stroke.
Keyword
MeSH Terms
-
Anoxia
Antimycin A
Blotting, Western
Brain
Carnosine*
Cell Survival
Enzyme-Linked Immunosorbent Assay
Infarction, Middle Cerebral Artery
Injections, Intraperitoneal
Ischemia*
Neuroblastoma
Neurons
Neuroprotective Agents*
Oxidative Stress
Reactive Oxygen Species
Rodentia*
Stroke
Superoxide Dismutase
Antimycin A
Carnosine
Neuroprotective Agents
Reactive Oxygen Species
Superoxide Dismutase
Figure
Cited by 1 articles
-
Neuroprotective Effect of Resveratrol on Acute Brain Ischemia Reperfusion Injury by Measuring Annexin V, p53, Bcl-2 Levels in Rats
Ceren Kizmazoglu, Hasan Emre Aydin, Ismail Ertan Sevin, Orhan Kalemci, Nurullah Yüceer, Metin Ant Atasoy
J Korean Neurosurg Soc. 2015;58(6):508-512. doi: 10.3340/jkns.2015.58.6.508.
Reference
-
1. Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004; 61:657–668. PMID: 15052409.
Article2. Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981; 12:723–725. PMID: 6272455.
Article3. Bonfanti L, Peretto P, De Marchis S, Fasolo A. Carnosine-related dipeptides in the mammalian brain. Prog Neurobiol. 1999; 59:333–353. PMID: 10501633.
Article4. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009; 40:e331–e339. PMID: 19182083.
Article5. Carmichael ST. Rodent models of focal stroke : size, mechanism, and purpose. NeuroRx. 2005; 2:396–409. PMID: 16389304.6. Chez MG, Buchanan CP, Aimonovitch MC, Becker M, Schaefer K, Black C, et al. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J Child Neurol. 2002; 17:833–837. PMID: 12585724.
Article7. De Marchis S, Melcangi RC, Modena C, Cavaretta I, Peretto P, Agresti C, et al. Identification of the glial cell types containing carnosine-related peptides in the rat brain. Neurosci Lett. 1997; 237:37–40. PMID: 9406874.
Article8. Decker EA, Ivanov V, Zhu BZ, Frei B. Inhibition of low-density lipoprotein oxidation by carnosine histidine. J Agric Food Chem. 2001; 49:511–516. PMID: 11305256.9. Dobrota D, Fedorova T, Stvolinsky S, Babusikova E, Likavcanova K, Drgova A, et al. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury : after-stroke-effect. Neurochem Res. 2005; 30:1283–1288. PMID: 16341589.
Article10. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000; 62:649–671. PMID: 10880854.
Article11. Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001; 128:263–276. PMID: 11718758.
Article12. Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995; 64:97–112. PMID: 7574505.
Article13. Ginsberg MD. Adventures in the pathophysiology of brain ischemia : penumbra, gene expression, neuroprotection : the 2002 Thomas Willis Lecture. Stroke. 2003; 34:214–223. PMID: 12511777.
Article14. Hipkiss AR, Preston JE, Himsworth DT, Worthington VC, Keown M, Michaelis J, et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann N Y Acad Sci. 1998; 854:37–53. PMID: 9928418.
Article15. Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994; 36:557–565. PMID: 7944288.
Article16. Jin CL, Yang LX, Wu XH, Li Q, Ding MP, Fan YY, et al. Effects of carnosine on amygdaloid-kindled seizures in Sprague-Dawley rats. Neuroscience. 2005; 135:939–947. PMID: 16125861.
Article17. Kim HY, Koh SH, Kim SH. Rat models for ischemic stroke. Korean J Stroke. 2011; 13:107–113.
Article18. Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. I : a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986; 8:1–8.19. Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology. 2008; 55:281–288. PMID: 18541276.
Article20. Li F, Omae T, Fisher M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke. 1999; 30:2464–2470. discussion 2470-2471. PMID: 10548685.
Article21. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20:84–91. PMID: 2643202.
Article22. Maciel EN, Vercesi AE, Castilho RF. Oxidative stress in Ca(2+)-induced membrane permeability transition in brain mitochondria. J Neurochem. 2001; 79:1237–1245. PMID: 11752064.
Article23. Markus R, Reutens DC, Kazui S, Read S, Wright P, Pearce DC, et al. Hypoxic tissue in ischaemic stroke : persistence and clinical consequences of spontaneous survival. Brain. 2004; 127(Pt 6):1427–1436. PMID: 15130953.
Article24. Min J, Senut MC, Rajanikant K, Greenberg E, Bandagi R, Zemke D, et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res. 2008; 86:2984–2991. PMID: 18543335.
Article25. Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009; 109(Suppl 1):133–138. PMID: 19393019.
Article26. Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, et al. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010; 1802:92–99. PMID: 19751828.
Article27. Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996; 27:327–331. discussion 332. PMID: 8571432.
Article28. Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007; 2:219–236. PMID: 18044138.29. Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, et al. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007; 38:3023–3031. PMID: 17916766.
Article30. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661. PMID: 17942826.
Article31. Stellingwerff T, Decombaz J, Harris RC, Boesch C. Optimizing human in vivo dosing and delivery of β-alanine supplements for muscle carnosine synthesis. Amino Acids. 2012; 43:57–65. PMID: 22358258.
Article32. Stvolinsky SL, Dobrota D. Anti-ischemic activity of carnosine. Biochemistry (Mosc). 2000; 65:849–855. PMID: 10951104.33. Woo KJ. Annual report on the cause of death statistics 2010. Daejeon: Korea National Statistical Office;2010. p. 9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effect of Focal Ischemic Preconditioning in Transient Focal Ischemia Animal Model
- Effects of carnosine and hypothermia combination therapy on hypoxic-ischemic brain injury in neonatal rats
- L-histidine and L-carnosine exert anti-brain aging effects in D-galactose-induced aged neuronal cells
- Quantitative Analysis of Agmatine by HPLC in Ischemic Brain
- Diverse Ischemic Postconditioning Protocols Affect the Infarction Size in Focal Ischemic Stroke