J Cerebrovasc Endovasc Neurosurg.
2018 Sep;20(3):159-167. 10.7461/jcen.2018.20.3.159.
Diverse Ischemic Postconditioning Protocols Affect the Infarction Size in Focal Ischemic Stroke
- Affiliations
-
- 1Department of Neurosurgery, Gwangju Christian Hospital, Gwangju, Korea.
- 2Department of Neurosurgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea. nsjsp@chonnam.ac.kr
- KMID: 2422767
- DOI: http://doi.org/10.7461/jcen.2018.20.3.159
Abstract
OBJECTIVE
Ischemic postconditioning (IPostC), consisted of transient brain ischemia/reperfusion cycles, is considered to have neuroprotective effect. However, there is no best single protocol of IPostC, because varied factors like species tested and characteristics of the tissue may affect the efficacy of IPostC. Thus, we investgated whether different protocols of IPostC affect neuroprotective effects in experimental animal models.
MATERIALS AND METHODS
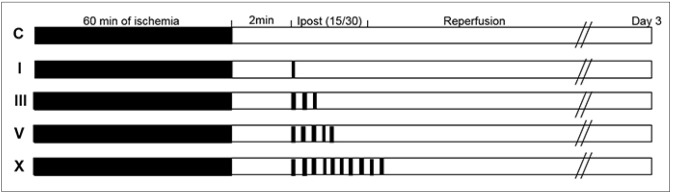
Through occlusion of middle cerebral artery (MCA) with intraluminal suture, stroke was induced in a transient focal ischemia model in mice. We conducted IPostC via brief and repeated MCA occlusion, 2 minutes after reperfusion, followed by different ischemia and reperfusion protocols. After procedure, functional neurological score and histological examination were evaluated.
RESULTS
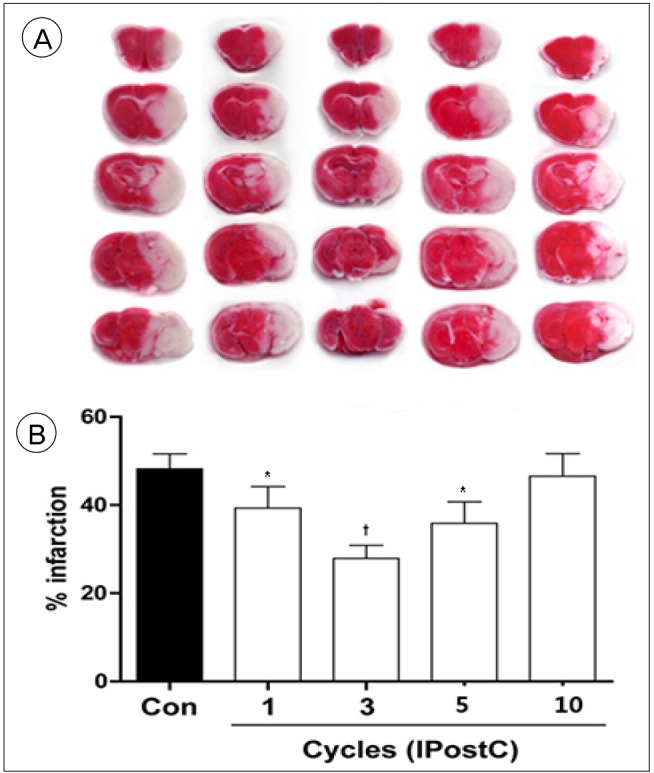
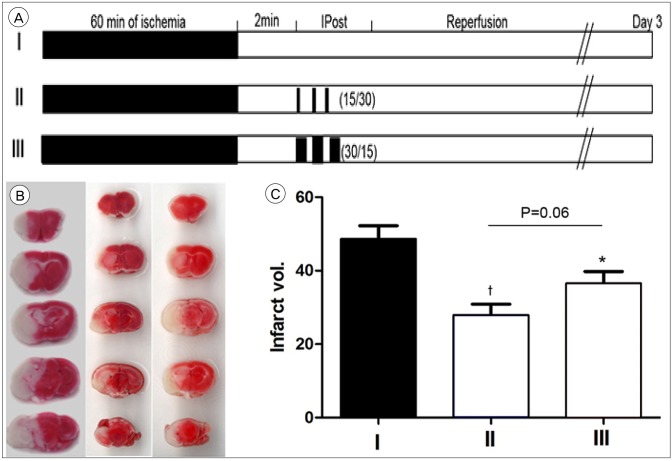
IPostC with different protocols resulted in diverse effects. Among them, a protocol that consists of 3 cycle of IPostC significantly reduced the infarction size 3 days after stroke.
CONCLUSION
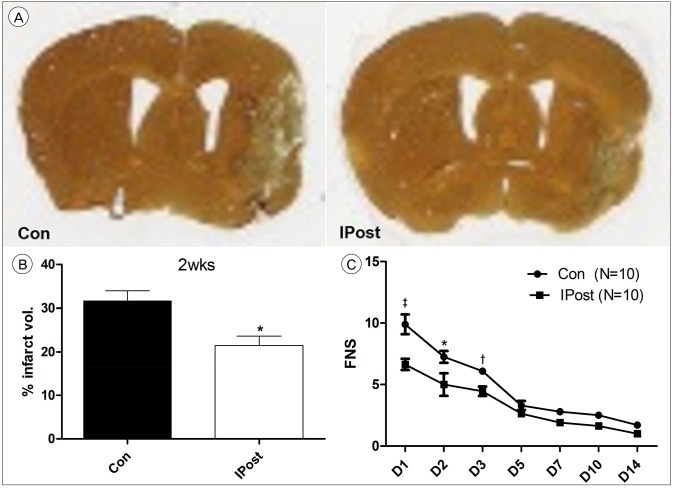
IPostC was confirmed to reduce infarction size. The effects of IPostC are definitely affected by differences in the protocol used, including the number of cycles, the duration of individual ischemia/reperfusion episode and the entire duration of the IPostC stimuli.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Systemic macrophage depletion attenuates infarct size in an experimental mouse model of stroke
Seung-Won Lee, Dong-Jun Song, Han-Seung Ryu, You-Sub Kim, Tae-Sun Kim, Sung-Pil Joo
J Cerebrovasc Endovasc Neurosurg. 2021;23(4):304-313. doi: 10.7461/jcen.2021.E2021.04.003.
Reference
-
1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017; 3. 135(10):e146–e603. PMID: 28122885.
Article2. Bretz B, Blaze C, Parry N, Kudej RK. Ischemic postconditioning does not attenuate ischemia-reperfusion injury of rabbit small intestine. Vet Surg. 2010; 2. 39(2):216–223. PMID: 20210969.3. Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008; 7. 27(7):1451–1474. PMID: 18275256.
Article4. Chu W, Li S, Wang S, Yan A, Nie L. Ischemic postconditioning provides protection against ischemia-reperfusion injury in intestines of rats. Int J Clin Exp Pathol. 2015; 6. 8(6):6474–6481. PMID: 26261524.5. Cohen MV, Yang XM, Downey JM. Conscious rabbits become tolerant to multiple episodes of ischemic preconditioning. Circ Res. 1994; 5. 74(5):998–1004. PMID: 8156647.
Article6. D'Amelio FE. The Golgi-Hortega-Lavilla technique, with a useful additional step for application to brain tissue after prolonged fixation. Stain Technol. 1983; 3. 58(2):79–84. PMID: 6194584.7. Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009; 4. 8(4):398–412. PMID: 19296922.
Article8. Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003; 5. 26(5):248–254. PMID: 12744841.
Article9. Doyle KP, Yang T, Lessov NS, Ciesielski TM, Stevens SL, Simon RP, et al. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J Cereb Blood Flow Metab. 2008; 6. 28(6):1235–1248. PMID: 18364727.
Article10. Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995; 9. 77(3):611–621. PMID: 7641331.
Article11. Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000; 4. 41(4):493–496. PMID: 10704275.12. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 9. 359(13):1317–1329. PMID: 18815396.
Article13. Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J. 2014; 1. 35(3):176–183. PMID: 24014392.
Article14. Iliodromitis EK, Kremastinos DT, Katritsis DG, Papadopoulos CC, Hearse DJ. Multiple cycles of preconditioning cause loss of protection in open-chest rabbits. J Mol Cell Cardiol. 1997; 3. 29(3):915–920. PMID: 9152852.
Article15. Joo SP, Xie W, Xiong X, Xu B, Zhao H. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013; 7. 23. 243:149–157. PMID: 23590905.
Article16. Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation. 1990; 8. 82(2):609–619. PMID: 2372907.
Article17. Lintz JA, Dalio MB, Joviliano EE, Piccinato CE. Ischemic pre and postconditioning in skeletal muscle injury produced by ischemia and reperfusion in rats. Acta Cir Bras. 2013; 6. 28(6):441–446. PMID: 23743682.
Article18. Papadopoulos D, Siempis T, Theodorakou E, Tsoulfas G. Hepatic ischemia and reperfusion injury and trauma: current concepts. Arch Trauma Res. 2013; 8. 2(2):63–70. PMID: 24396796.
Article19. Prasad A, Gössl M, Hoyt J, Lennon RJ, Polk L, Simari R, et al. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013; 5. 81(6):930–936. PMID: 22517646.
Article20. Sandhu R, Diaz RJ, Mao GD, Wilson GJ. Ischemic preconditioning: differences in protection and susceptibility to blockade with single-cycle versus multicycle transient ischemia. Circulation. 1997; 8. 96(3):984–995. PMID: 9264510.21. Santos CH, Gomes OM, Pontes JC, Miiji LN, Bispo MA. The ischemic preconditioning and postconditioning effect on the intestinal mucosa of rats undergoing mesenteric ischemia/reperfusion procedure. Acta Cir Bras. 2008; Jan-Feb. 23(1):22–28. PMID: 18278389.
Article22. Vogel J, Mobius C, Kuschinsky W. Early delineation of ischemic tissue in rat brain cryosections by high-contrast staining. Stroke. 1999; 5. 30(5):1134–1141. PMID: 10229755.
Article23. Whittaker P, Przyklenk K. From ischemic conditioning to ‘hyperconditioning’: clinical phenomenon and basic science opportunity. Dose Response. 2014; 12. 12(4):650–663. PMID: 25552962.
Article24. Xiong X, Gu L, Zhang H, Xu B, Zhu S, Zhao H. The protective effects of T cell deficiency against brain injury are ischemic model-dependent in rats. Neurochem Int. 2013; 2. 62(3):265–270. PMID: 23228347.
Article25. Zhao H. Hurdles to clear before clinical translation of ischemic postconditioning against stroke. Transl Stroke Res. 2013; 2. 4(1):63–70. PMID: 23524538.
Article26. Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009; 5. 29(5):873–885. PMID: 19240739.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ischemic postconditioning may not influence early brain injury induced by focal cerebral ischemia/reperfusion in rats
- Selective Gray Matter Infarction in the Basal Ganglia Associated With Transient Ischemic Attack
- Obesity as a Limiting Factor for Remote Ischemic Postconditioning-Mediated Neuroprotection after Stroke
- Pharmacological Secondary Prevention of Ischemic Stroke
- Multiple Territory Ischemic Stroke Aggravated by Severe Anemia