J Korean Med Assoc.
2009 Jul;52(7):638-644. 10.5124/jkma.2009.52.7.638.
Introduction to Autoimmune Disease
- Affiliations
-
- 1Department of Microbiology and Immunology, Kangwon National University College of Medicine, Korea. jchoe@kangwon.ac.kr
- KMID: 2188024
- DOI: http://doi.org/10.5124/jkma.2009.52.7.638
Abstract
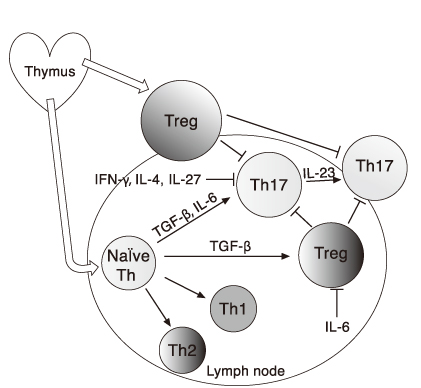
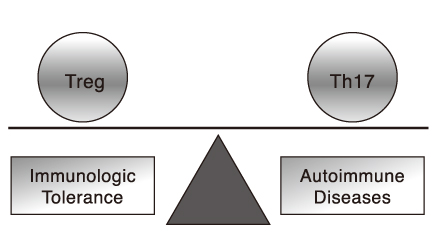
- The immune system maintains the integrity of our bodies by warding off intruding microorganisms, but by sustaining tolerance to our own tissues. The immunologic tolerance is established by several layers of safeguards, including physical elimination of self-reactive lymphocytes during their development in the central lymphoid organs, anergy induction in autoreactive lymphocytes before their emigration to the periphery, or production regulatory T lymphocytes that suppress the activation, proliferation, and differentiation of various effector cells. The major regulatory T lymphocytes display their phenotype as CD4(+)CD25(+)Foxp3(+) and constitute about 10% of the peripheral T lymphocytes. Even with these safeguards, the immunologic tolerance sometimes fails and generates autoimmune diseases. Scientists studying the pathogenesis of autoimmune diseases pay particular attention to a CD4(+) T lymphocytes subset, Th17 lymphocytes, distinct from Th1 and Th2. Th17 produces diverse proinflammatory cytokines including IL-17 and TNF-alpha. Th17 and these cytokines are causatively associated with many episodes of autoimmune diseases. Accumulated data reveal the critical role of Th17 cells in the pathology of autoimmunity and portray them as an important target in the treatment of various autoimmune diseases. In this article, we will describe the main characteristics of regulatory T cells and Th17 cells and their cellular and molecular mechanisms of protective or destructive functions, respectively.
MeSH Terms
Figure
Cited by 1 articles
-
FOXP3+T Cells and TGF-β1 in Colonic Mucosa of Children with Crohn's Disease
Joo Hyun Gil, Jung Eun Oh, Jeong Wan Seo, Min-Sun Cho, Ky Young Cho, Eun Sun Yoo
Korean J Pediatr Gastroenterol Nutr. 2011;14(3):258-268. doi: 10.5223/kjpgn.2011.14.3.258.
Reference
-
1. Pulendran B, van Driel R, Nossal GJV. Immunological tolerance in germinal centres. Immunol Today. 1997. 18:27–32.
Article2. Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000. 101:455–458.3. Carneiro-Sampaio M, Coutinho A. Tolerance and autoimmunity: lessons at the bedside of primary immunodeficiencies. Adv Immunol. 2007. 95:51–82.4. Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct Tcell subclasses. J Exp Med. 1976. 143:1391–1400.
Article5. Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in posTthymectomy autoimmune oophoritis in mice. II. Requirement of LyT1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982. 156:1577–1586.
Article6. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. 155:1151–1164.7. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008. 133:775–787.
Article8. Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory Tcell acativation and expansion. J Allergy Clin Immunol. 2009. 123:749–755.
Article9. Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999. 162:5317–5326.10. Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004. 114:1640–1649.
Article11. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory Tcell function. Nature. 2007. 450:566–569.
Article12. Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000. 12:431–440.
Article13. Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000. 192:295–302.
Article14. Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008. 105:10113–10118.
Article15. Tang Q, Bluestone JA. Regulatory Tcell physiology and application to treat autoimmunity. Immunol Rev. 2006. 212:217–237.
Article16. Belkaid Y, Tarbell KV. Arming Treg cells at the inflammatory site. Immunity. 2009. 30:322–323.
Article17. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986. 136:2348–2357.18. Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007. 6:169–175.
Article19. Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000. 165:6107–6115.
Article20. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro-and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003. 198:1951–1957.
Article21. Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the Th17 lineage. Nature. 2006. 441:231–234.
Article22. Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL) -1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006. 203:1685–1691.
Article23. Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Rev Immunol. 2006. 7:929–936.
Article24. Voo KS, Wang Y-H, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009. 106:4793–4798.25. Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007. 317:256–260.
Article26. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3+ regulatory T cells differentiate into IL-17-producing cells. Blood. 2008. 112:2340–2352.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclosporine Treatment in a Patient with Concurrent Autoimmune Urticaria and Autoimmune Hepatitis
- A case of Kikuchi-Fujimoto disease with autoimmune thyroiditis
- Autoimmune Thyroiditis during Antiviral Therapy with Peginterferon
- A case of membranoproliferative glomerulonephritis in a patient with Graves' disease
- Epidemiology of Autoimmune Liver Disease