J Korean Endocr Soc.
2007 Feb;22(1):35-44. 10.3803/jkes.2007.22.1.35.
Effects of Wnt-1 on the Growth and Apoptosis of FRTL-5 Cells
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Korea.
- 2Asan Institute for Life Sciences, University of Ulsan College of Medicine, Korea.
- KMID: 2178240
- DOI: http://doi.org/10.3803/jkes.2007.22.1.35
Abstract
-
BACKGROUND: Wnt proteins are major signaling molecules involved in embryonic induction, generation of cell polarity and the cell fate decision. A central player in the Wnt signaling pathways is beta-catenin. Several studies have suggested that the Wnt/beta-catenin signaling pathway may be involved in the physiologic/pathologic control of thyroid cell growth and function.
METHODS
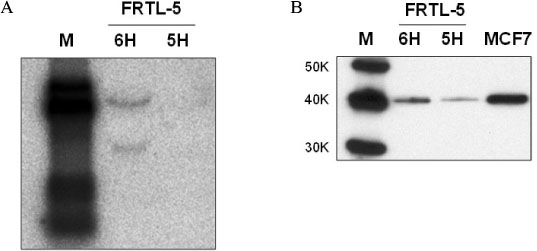
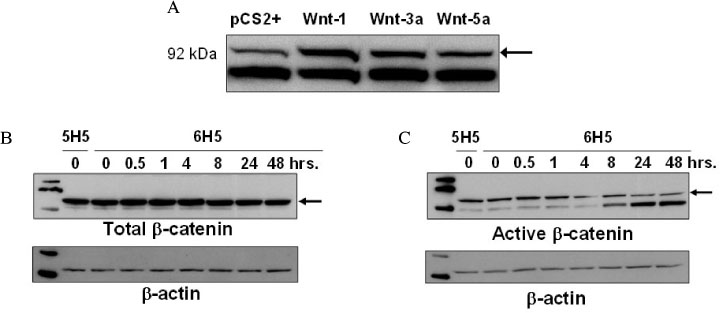
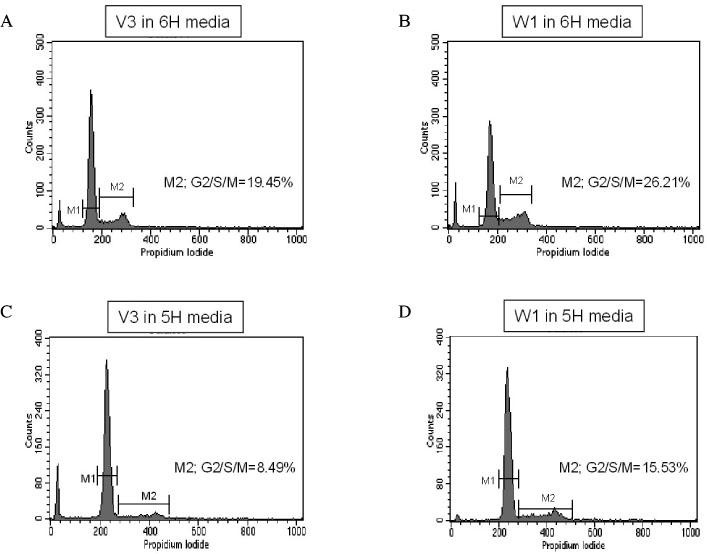
We investigated the effect of thyroid-stimulating hormone (TSH) on the expression of Wnt proteins in FRTL-5 cells. To evaluate the effect of Wnt-1 on FRTL-5 cells growth, we isolated a stable cell line that overexpressed Wnt-1 (W1), and a vector-transfected cell clone (V3) was used as a control. We investigated the differences in the cellular growth rate, the cell cycle and cell apoptosis in the W1 and V3 cell lines.
RESULTS
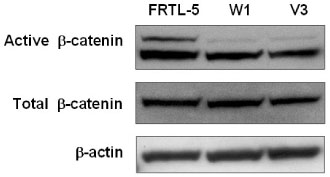
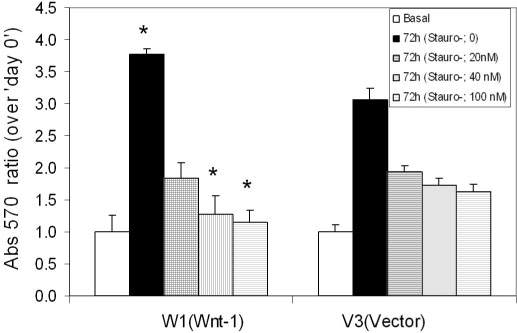
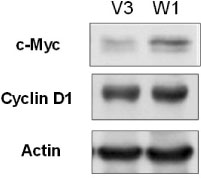
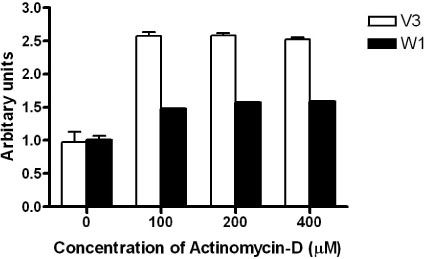
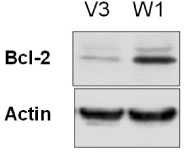
TSH caused a significant increase in the Wnt-1 level and a pronounced decrease in both the active and total beta-catenin levels in the FRTL-5 cells. The growth rate, the percentage of cells in the S/G2/M phase and the c-myc level were significantly higher in the W1 cells compared with the V3 cells. There was no change in the beta-catenin level and the cyclin D1 level in the W1 cells compared with the V3 cells. The cellular apoptosis induced by actinomycin-D seemed to be significantly decreased because the level of bcl-2 was increased in the W1 cells compared with the V3 cells.
CONCLUSION
The FRTL-5 cells expressed Wnt-1 protein, and TSH increased the Wnt-1 expression, and it paradoxically decreased beta-catenin in the FRTL-5 cells. Overexpression of Wnt-1 in the FRTL-5 cells increased cell growth and it decreased apoptosis. Growth stimulation by Wnt-1 overexpression was not mediated by beta-catenin (the canonical Wnt pathway), but seemed to be mediated by activation of the Wnt/Ca2+ pathway, which involves an increased c-myc level. Suppression of apoptosis with Wnt-1 overexpression was due to the increased bcl-2 level.
Keyword
MeSH Terms
Figure
Reference
-
1. Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997. 11:3286–3305.2. Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004. 5:691–701.3. Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004. 303:1483–1487.4. Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002. 4:E101–E108.5. Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000. 148:399–404.6. Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999. 18:7860–7872.7. Polakis P. Wnt signaling and cancer. Genes Dev. 2000. 14:1837–1851.8. Polakis P. Casein kinase 1: a Wnt'er of disconnect. Curr Biol. 2002. 12:R499–R501.9. Morin PJ. Beta-catenin signaling and cancer. Bioessays. 1999. 21:1021–1030.10. Webster MT, Rozycka M, Sara E, Davis E, Smalley M, Young N, Dale TC, Wooster R. Sequence variants of the axin gene in breast, colon, and other cancers: an analysis of mutations that interfere with GSK3 binding. Genes Chromosomes Cancer. 2000. 28:443–453.11. Clevers H. Axin and hepatocellular carcinomas. Nat Genet. 2000. 24:206–208.12. Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997. 1332:F127–F147.13. Helmbrecht K, Kispert A, von WR, Brabant G. Identification of a Wnt/beta-catenin signaling pathway in human thyroid cells. Endocrinology. 2001. 142:5261–5266.14. Cerrato A, Fulciniti F, Avallone A, Benincasa G, Palombini L, Grieco M. Beta- and gamma-catenin expression in thyroid carcinomas. J Pathol. 1998. 185:267–272.15. Huang SH, Wu JC, Chang KJ, Liaw KY, Wang SM. Expression of the cadherin-catenin complex in well-differentiated human thyroid neoplastic tissue. Thyroid. 1999. 9:1095–1103.16. Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999. 59:1811–1815.17. Garcia-Rostan G, Camp RL, Herrero A, Carcangiu ML, Rimm DL, Tallini G. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol. 2001. 158:987–996.18. Kohn LD, Saji M, Akamizu T, Ikuyama S, Isozaki O, Kohn AD, Santisteban P, Chan JY, Bellur S, Rotella CM. Receptors of the thyroid: the thyrotropin receptor is only the first violinist of a symphony orchestra. Adv Exp Med Biol. 1989. 261:151–209.19. Bidey SP, Lambert A, Robertson WR. Thyroid cell growth, differentiation and function in the FRTL-5 cell line: a survey. J Endocrinol. 1988. 119:365–376.20. Kikkawa F, Gonzalez FJ, Kimura S. Characterization of a thyroid-specific enhancer located 5.5 kilobase pairs upstream of the human thyroid peroxidase gene. Mol Cell Biol. 1990. 10:6216–6224.21. Shimura H, Okajima F, Ikuyama S, Shimura Y, Kimura S, Saji M, Kohn LD. Thyroid-specific expression and cyclic adenosine 3',5'-monophosphate autoregulation of the thyrotropin receptor gene involves thyroid transcription factor-1. Mol Endocrinol. 1994. 8:1049–1069.22. Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996. 10:1443–1454.23. Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996. 6:1302–1306.24. McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989. 58:1075–1084.25. Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A. 2004. 101:12682–12687.26. Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, Krause PR, Kohn LD. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci U S A. 1999. 96:2285–2290.27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.28. van NM, van de WM, Clevers H. Identification of two novel regulated serines in the N terminus of beta-catenin. Exp Cell Res. 2002. 276:264–272.29. Lu Z, Hunter T. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle. 2004. 3:571–573.30. Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999. 398:422–426.31. Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999. 96:5522–5527.32. He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998. 281:1509–1512.33. Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002. 2:764–776.34. Isozaki O, Kohn LD. Control of c-fos and c-myc proto-oncogene induction in rat thyroid cells in culture. Mol Endocrinol. 1987. 1:839–848.35. Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999. 9:695–698.36. Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997. 390:410–413.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Estrogen on H2O2 Induced Apoptosis of FRTL-5 Cells
- Effect of ceramide on apoptosis and phospholipase D activity in FRTL-5 thyroid cells
- Pseudolaric acid B inhibits PAX2 expression through Wnt signaling and induces BAX expression, therefore promoting apoptosis in HeLa cervical cancer cells
- Overexpression of USF Increases TGF-beta1 Protein Levels, But G1 Phase Arrest was not Induced in FRTL-5 Cells
- Effect of Lithium on Na+/I- Symporter Gene Expression in Rat Thyroid FRTL-5 Cells