J Cardiovasc Ultrasound.
2010 Sep;18(3):91-97. 10.4250/jcu.2010.18.3.91.
Targeted Ultrasound Imaging of Apoptosis with Annexin A5 Microbubbles in Acute Doxorubicin-Induced Cardiotoxicity
- Affiliations
-
- 1Division of Cardiology, Heart Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 2Yonsei Cardiovascular Center and Research Institute, Yonsei University College of Medicine, Seoul, Korea. namsikc@yuhs.ac
- 3Biobud Co. Ltd, Seoul, Korea.
- 4Department of Biochemistry, CHA University College of Medicine, Sungnam, Korea.
- KMID: 2177306
- DOI: http://doi.org/10.4250/jcu.2010.18.3.91
Abstract
- BACKGROUND
The aim of this study was to assess the feasibility of targeted ultrasound imaging on apoptosis with annexin A5 microbubbles (A5MB) in acute doxorubicin-induced cardiotoxicity.
METHODS
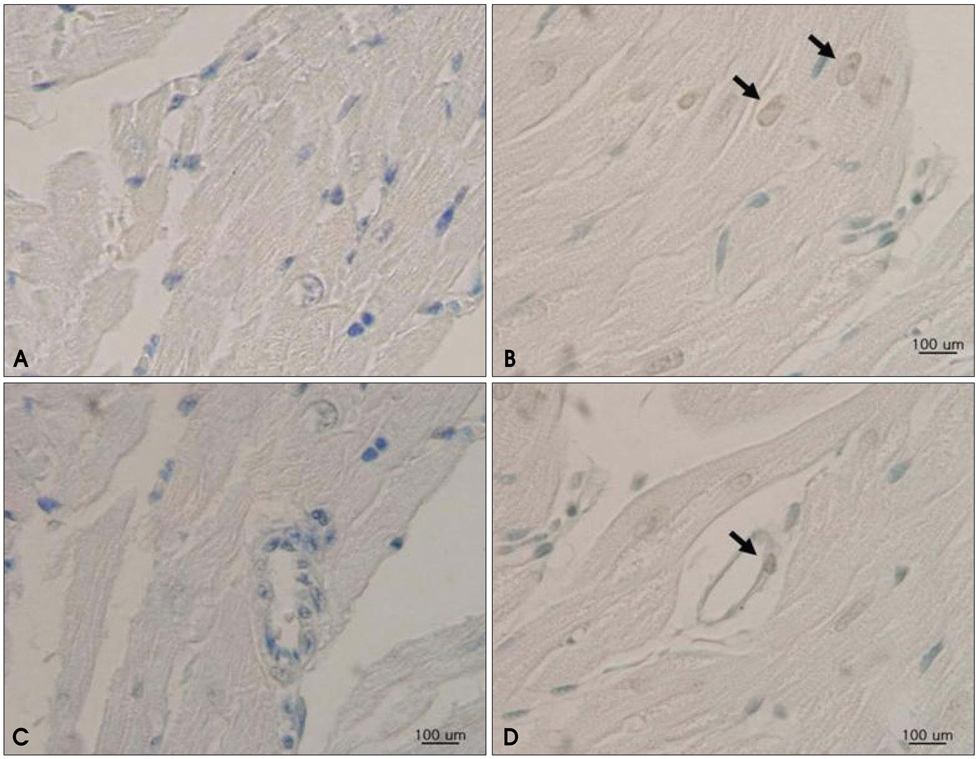
Avidinated and octafluoropropan-filled phospholipid microbubbles were conjugated with biotinylated annexin A5. To confirm the specific binding of A5MB, flow cytometry was performed with hydrogen peroxide induced apoptosis in rat aorta smooth muscle cells incubated with fluorescein-5-isothiocyanate (FITC) labeled annexin A5 and A5MB. Adult male rats were injected intraperitoneally with 5 mg/kg doxorubicin weekly for 3 weeks (n = 5). Control rats were injected with normal saline (n = 5). At 24 hours after the final treatment, triggering imaging was performed 15 min after an intravenous bolus injection of A5MB for washout of freely circulating microbubbles. After echocardiography, the heart was isolated for histological detection of apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay.
RESULTS
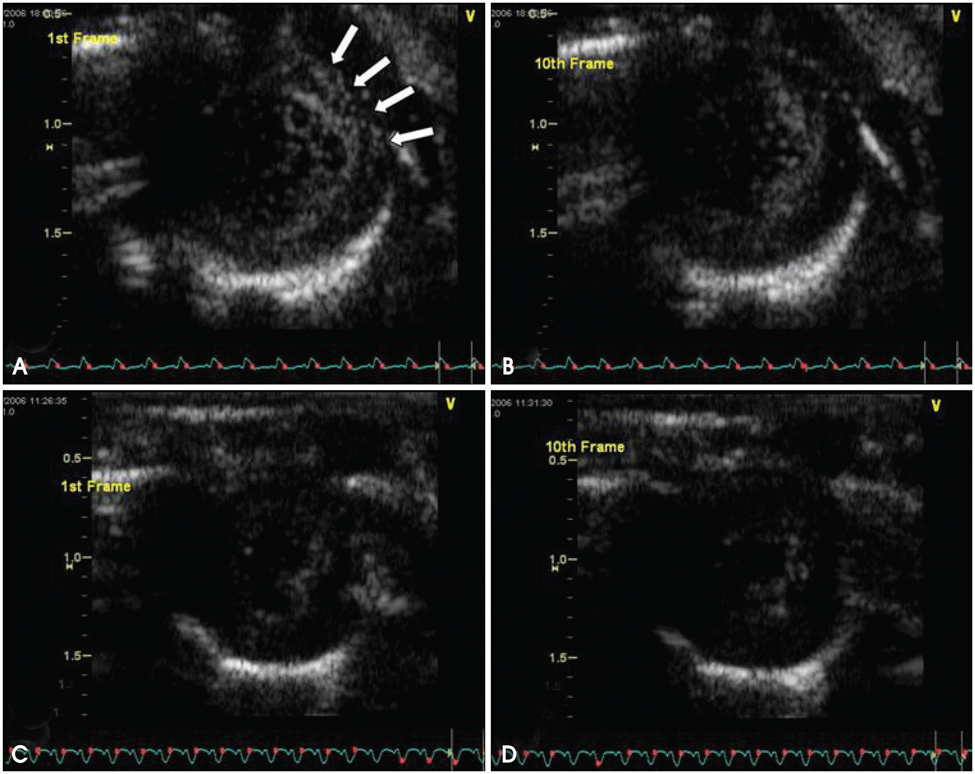
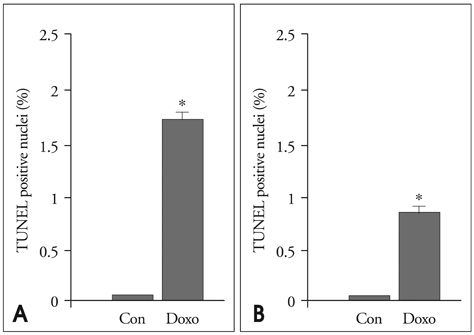
In the in vitro tests, fluorescence intensity was low for healthy cells and high for apoptotic cells when incubated with FITC-labeled annexin A5 and A5MB. Rats treated with doxorubicin showed significant contrast opacification of the myocardium on contrast echocardiography using A5MB. However, no opacification was observed in control rats. Apoptosis was confirmed by TUNEL assay in doxorubicin treated rats.
CONCLUSION
Acute doxorubicin-induced cardiomyopathy based on early apoptosis can be assessed and imaged with targeted ultrasound imaging using A5MB in rats.
Keyword
MeSH Terms
-
Adult
Animals
Annexin A5
Aorta
Apoptosis
Avidin
Cardiomyopathies
Doxorubicin
Echocardiography
Flow Cytometry
Fluorescein-5-isothiocyanate
Fluorescence
Heart
Humans
Hydrogen Peroxide
In Situ Nick-End Labeling
Male
Microbubbles
Myocardium
Myocytes, Smooth Muscle
Rats
Annexin A5
Avidin
Doxorubicin
Fluorescein-5-isothiocyanate
Hydrogen Peroxide
Figure
Reference
-
1. Bristow MR, Billingham ME, Mason JW, Daniels JR. Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat Rep. 1978. 62:873–879.2. Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002. 86:1697–1700.
Article3. Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997. 95:320–323.
Article4. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997. 336:1131–1141.
Article5. Saraste A, Pulkki K, Kallajoki M, Heikkilä P, Laine P, Mattila S, Nieminen MS, Parvinen M, Voipio-Pulkki LM. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest. 1999. 29:380–386.
Article6. Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation. 2000. 102:572–578.
Article7. Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000. 60:1789–1792.8. Ling YH, Priebe W, Perez-Soler R. Apoptosis induced by anthracycline antibiotics in P388 parent and multidrug-resistant cells. Cancer Res. 1993. 53:1845–1852.9. Zhang J, Clark JR Jr, Herman EH, Ferrans VJ. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. J Mol Cell Cardiol. 1996. 28:1931–1943.
Article10. van Heerde WL, Robert-Offerman S, Dumont E, Hofstra L, Doevendans PA, Smits JF, Daemen MJ, Reutelingsperger CP. Markers of apoptosis in cardiovascular tissues: focus on Annexin V. Cardiovasc Res. 2000. 45:549–559.
Article11. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998. 31:1–9.
Article12. Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007. 18:11–16.
Article13. Klibanov AL. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv Drug Deliv Rev. 1999. 37:139–157.
Article14. Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004. 11:215–221.
Article15. Bayne K. American Physiological Society. Revised Guide for the Care and Use of Laboratory Animals available. Physiologist. 1996. 39:199208–211.16. Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004. 94:433–445.17. Narula J, Acio ER, Narula N, Samuels LE, Fyfe B, Wood D, Fitzpatrick JM, Raghunath PN, Tomaszewski JE, Kelly C, Steinmetz N, Green A, Tait JF, Leppo J, Blankenberg FG, Jain D, Strauss HW. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001. 7:1347–1352.
Article18. Hofstra L, Liem IH, Dumont EA, Boersma HH, van Heerde WL, Doevendans PA, De Muinck E, Wellens HJ, Kemerink GJ, Reutelingsperger CP, Heidendal GA. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet. 2000. 356:209–212.
Article19. Dumont EA, Reutelingsperger CP, Smits JF, Daemen MJ, Doevendans PA, Wellens HJ, Hofstra L. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 2001. 7:1352–1355.
Article20. Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, Steinmetz N, van Eck-Smit BL. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004. 45:842–848.21. Wu S, Ko YS, Teng MS, Ko YL, Hsu LA, Hsueh C, Chou YY, Liew CC, Lee YS. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol. 2002. 34:1595–1607.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Auranofin Suppresses Plasminogen Activator Inhibitor-2 Expression through Annexin A5 Induction in Human Prostate Cancer Cells
- Role of Annexin A5 on Mitochondria-Dependent Apoptosis Induced by Tetramethoxystilbene in Human Breast Cancer Cells
- In Vivo Nuclear Imaging of Apoptosis
- Future Applications of Contrast Ultrasound
- Restoration of the TRAIL Resistance with a Low Dose Doxorubicin in Acute Leukemia Cell Lines