J Breast Cancer.
2007 Sep;10(3):180-192. 10.4048/jbc.2007.10.3.180.
A Study for Expression and Biological Function of N-myc Downstream Regulated Gene 2 in Breast Cancer
- Affiliations
-
- 1Division of Breast-Endocrine Surgery, Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea. shjung@chonbuk.ac.kr
- KMID: 2175034
- DOI: http://doi.org/10.4048/jbc.2007.10.3.180
Abstract
-
PURPOSE: It is important to identify a potential tumor marker that is associated with pathophysiologic processes of breast cancer. N-Myc downstream regulated genes (NDRG) are composed of four subtypes (NDRG 1-4) and NDRG2 gene has been reported as a specifically expressed gene in the human solid tumor including breast cancer. Although NDRG2 inhibits cell proliferation and promote differentiation, the molecular basis of the tumor-suppressor activity of NDRG2 in breast cancer is unknown. Herein, we tried to reveal the correlations between the expression of NDRG2 and the various clinicopathologic prognostic factors and evaluate its functional and pathophysiological roles in tumorigenesis of breast cancer.
METHODS
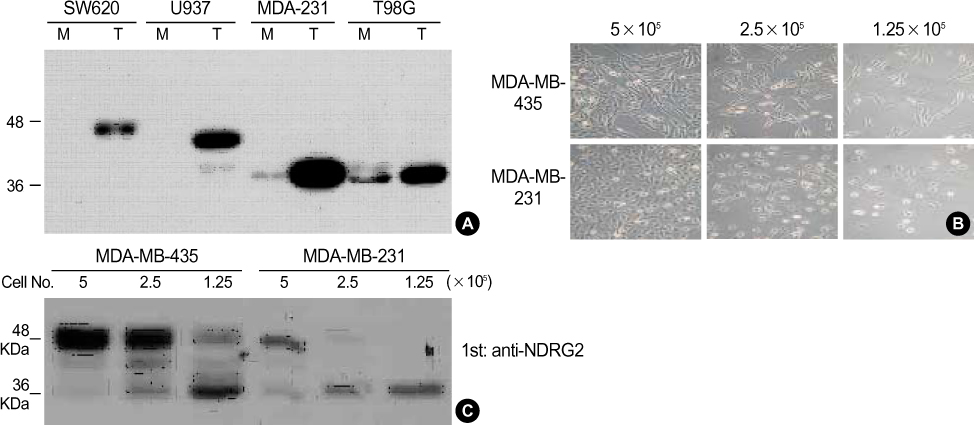
We were obtained the 67 breast cancers and paired normal tissue samples from patients who operated for breast cancer between June 2002 and June 2004. The expression of NDRG2 were measured with immunohistochemistry using monoclonal antibody and it was used eukaryotic transfection to manipulate the expression in MDA-MB-231 breast cancer cell line. Cell proliferation analysis were evaluated with trypan blue stain and status of differentially-expressed genes by NDRG2 overexpression were investigated with oligo microarray chip analysis.
RESULTS
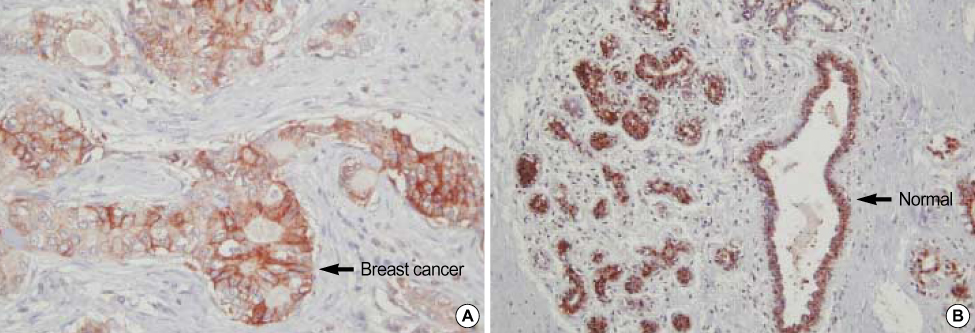
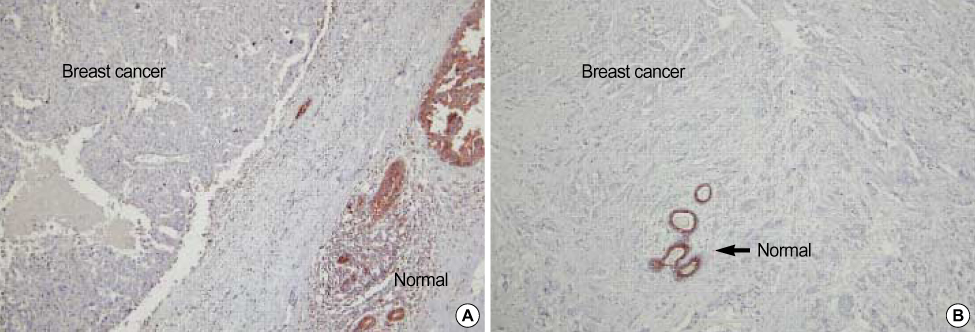
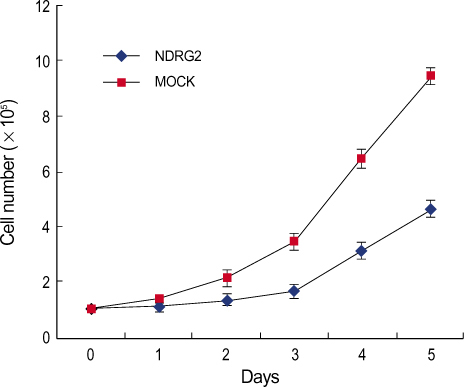
Significant difference of NDRG2 mRNA expression between breast cancer and normal tissue was not detected. However, NDRG2 was significantly down-regulated in breast cancer tissue, compared to normal tissue (p<0.0001). It was a inverse-correlation between the NDRG2 expression and tumor size, histologic grade although other clinicopathological parameters such as axillary lymph node metastasis were not correlated. Overexpression of NDRG2 in MDA-MB-231 cell showed a decrease of cell proliferation compared with Mock control. Of the 24,000 genes, 64 genes were increased in expression while 256 genes including cyclin D1 were repressed by NDRG2 overexpression.
CONCLUSION
Our results suggest that NDRG2 can function as a regulator of cell differentiation and cell cycle (as a tumor suppressor gene) in the early stage of breast cancer. In addition, NDRG2 protein indicates a prognostic tumor marker for breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Lauren J, Gunji Y, Alitalo K. Is angiopoietin-2 necessary for the initiation of tumor angiogenesis? Am J Pathol. 1998. 153:1459–1466.2. Wallden B, Emond M, Swift ME, Disis ML, Swisshelm K. Antimetastatic gene expression profiles mediated by retinoic acid receptor beta 2 in MDA-MB-435 breast cancer cells. BMC Cancer. 2005. 5:140.
Article3. Choi DH, Kim S, Rimm DL, Carter D, Haffty BG. Immunohistochemical biomarkers in patients with early-onset breast carcinoma by tissue microarray. Cancer J. 2005. 11:404–411.
Article4. Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989. 63:181–187.
Article5. McGuckin MA, Cummings MC, Walsh MD, Hohn BG, Bennett IC, Wright RG. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer. 1996. 73:88–95.
Article6. Kalaydjieva L, Gresham D, Gooding R, Heather L, Baas F, de Jonge R, et al. N-myc downstream-regulated gene 1 is mutated in hereditary motor and sensory neuropathy-Lom. Am J Hum Genet. 2000. 67:47–58.
Article7. Shimono A, Okuda T, Kondoh H. N-myc-dependent repression of ndr1, a gene identified by direct subtraction of whole mouse embryo cDNAs between wild type and N-myc mutant. Mech Dev. 1999. 83:39–52.
Article8. Kurdistani SK, Arizti P, Reimer CL, Sugrue MM, Aaronson SA, Lee SW. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res. 1998. 58:4439–4444.9. Okuda T, Kondoh H. Identification of new genes ndr2 and ndr3 which are related to Ndr1/RTP/Drg1 but show distinct tissue specificity and response to N-myc. Biochem Biophys Res Commun. 1999. 266:208–215.
Article10. Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, et al. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003. 106:342–347.
Article11. Choi SC, Kim KD, Kim JT, Kim JW, Yoon DY, Choe YK, et al. Expression and regulation of NDRG2 (N-myc downstream regulated gene 2) during the differentiation of dendritic cells. FEBS Lett. 2003. 553:413–418.
Article12. Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y, et al. Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol Cell Biochem. 2002. 229:35–44.13. Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002. 94:2511–2516.
Article14. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987. 162:156–159.
Article15. Gershoni JM, Palade GE. Protein blotting: principles and applications. Anal Biochem. 1983. 131:1–15.
Article16. Yang SH, Kim JS, Oh TJ, Kim MS, Lee SW, Woo SK, et al. Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray. Int J Oncol. 2003. 22:741–750.
Article17. Lusis EA, Watson MA, Chicoine MR, Lyman M, Roerig P, Reifenberger G, et al. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005. 65:7121–7126.
Article18. Shaw E, McCue LA, Lawrence CE, Dordick JS. Identification of a novel class in the alpha/beta hydrolase fold superfamily: the N-myc differentiation-related proteins. Proteins. 2002. 47:163–168.
Article19. Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001. 73:86–97.
Article20. Boulkroun S, Fay M, Zennaro MC, Escoubet B, Jaisser F, Blot-Chabaud M, et al. Characterization of rat NDRG2 (N-Myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J Biol Chem. 2002. 277:31506–31515.
Article21. Burchfield JG, Lennard AJ, Narasimhan S, Hughes WE, Wasinger VC, Corthals GL, et al. Akt mediates insulin-stimulated phosphorylation of Ndrg2: evidence for cross-talk with protein kinase C theta. J Biol Chem. 2004. 279:18623–18632.22. Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998. 332:281–292.
Article23. Jaken S, Parker PJ. Protein kinase C binding partners. BioEssays. 2000. 22:245–254.
Article24. Schmitz-Peiffer C. Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Ann N Y Acad Sci. 2002. 967:146–157.
Article25. Boulkroun S, Fay M, Zennaro MC, Escoubet B, Jaisser F, Blot-Chabaud M, et al. Characterization of rat NDRG2 (N-Myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J Biol Chem. 2002. 277:31506–31515.
Article26. Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, et al. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA. 1999. 96:2514–2519.
Article27. Lusis EA, Watson MA, Chicoine MR, Lyman M, Roerig P, Reifenberger G, et al. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005. 65:7121–7126.
Article28. Kim YH, PetKo Z, Dzieciatkowski S, Lin L, Ghiassi M, Stain S, et al. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006. 45:781–789.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- c-Myc-Induced Long Non-Coding RNA Small Nucleolar RNA Host Gene 7 Regulates Glycolysis in Breast Cancer
- Change of Mad1 Expression in Human Breast Cancer and Normal Breast Tissues
- Expression of Multidrug Resistance-associated Protein(MRP), c-myc and c-fos in L1210 Cells
- Erratum: c-Myc-Induced Long Non-Coding RNA SNHG7 Regulates Glycolysis in Breast Cancer
- Gene Expression Profiling of Breast Cancers with Emphasis of beta-Catenin Regulation