J Korean Med Sci.
2004 Apr;19(2):275-282. 10.3346/jkms.2004.19.2.275.
Gene Expression Profiling of Breast Cancers with Emphasis of beta-Catenin Regulation
- Affiliations
-
- 1Department of Pathology, Dong-A University College of Medicine, Busan, Korea. msroh@netian.com
- 2Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Surgery, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Urology, Dong-A University College of Medicine, Busan, Korea.
- 5Department of Pharmacology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 1733489
- DOI: http://doi.org/10.3346/jkms.2004.19.2.275
Abstract
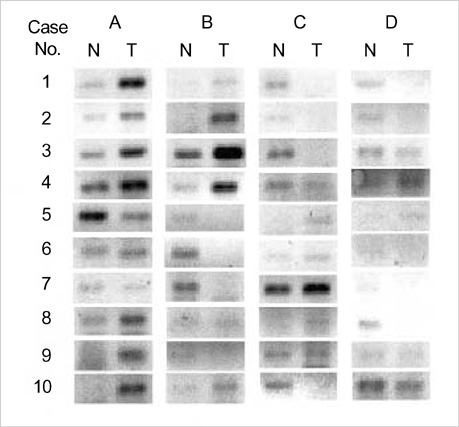
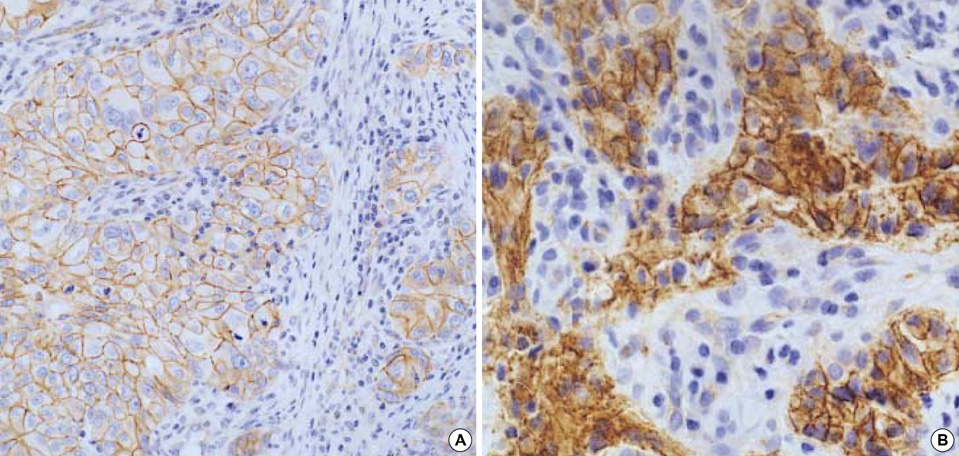
- To gain molecular understanding of carcinogenesis of breast cancer, gene expression profiles were analyzed using cDNA microarray representing 4,600 cDNAs in 10 breast cancer samples and the adjacent noncancerous breast tissues from the same patients. The alterations in gene expression levels were confirmed by reversetranscription PCR in four randomly selected genes. Genes that were differently expressed in cancer and noncancerous tissues were identified. 106 (of which 55 were known) and 49 (of which 28 were known) genes were up- or down-regulated, respectively, in greater than 60% of the breast cancer samples. In cancer tissues, genes related to cell cycle, transcription, metabolism, cell structure/motility and signal transduction were mostly up-regulated. Furthermore, three cancer tissues showing immunohistochemically aberrant accumulation of beta-catenin in the nucleus and/or cytoplasm revealed down-regulation of Siah and Axin genes and up-regulation of Wnt and c-myc genes. These findings were highly consistent with Wnt signaling pathway associated with beta-catenin regulation previously suggested by others. Our studies, therefore, provide not only a molecular basis to understand biological processes of breast cancer but also useful resources to define the mechanism of beta-catenin expression in tumorigenesis of breast cancer.
Keyword
MeSH Terms
-
Adult
Breast Neoplasms/*genetics/*metabolism/pathology
Cytoskeletal Proteins/*metabolism
Female
*Gene Expression Profiling/standards
Gene Expression Regulation, Neoplastic
Human
Immunohistochemistry
Middle Aged
*Oligonucleotide Array Sequence Analysis/standards
Reproducibility of Results
Reverse Transcriptase Polymerase Chain Reaction
Signal Transduction
Support, Non-U.S. Gov't
Trans-Activators/*metabolism
Figure
Reference
-
1. Shalon D, Smith SJ, Brown PO. A DNA microarray system for analyzing complex DNA samples using two-color fluorescent probe hybridization. Genome Res. 1996. 6:639–645.
Article2. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001. 98:10869–10874.
Article3. van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002. 415:530–536.4. Jenssen TK, Kuo WP, Stokke T, Hovig E. Associations between gene expressions in breast cancer and patient survival. Hum Genet. 2002. 111:411–420.5. van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AAM, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002. 347:1999–2009.
Article6. Huang E, Cheng SH, Dressman H, Pittman J, Tsou MH, Horng CF, Bild A, Iversen ES, Liao M, Chen CM, West M, Nevins JR, Huang AT. Gene expression predictors of breast cancer outcomes. Lancet. 2003. 361:1590–1596.
Article7. Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A, Ferno M, Peterson C, Meltzer PS. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001. 61:5979–5984.8. Kim HS, Jung JH, Park HY, Lee YH, Chung EJ, Kim MK, Kim JC. Gene expression profile analysis of human breast cancer using cDNA microarrays. J Korean Breast Cancer Soc. 2003. 6:58–67.9. Han W, Chung KW, Ahn SJ, Noh DY, Youn YK, Oh SK, Choe KJ. Gene expression profiles of primary breast cancer tissue using cDNA microarray. J Korean Breast Cancer Soc. 2002. 5:284–290.
Article10. Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. β-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 1999. 97:4262–4266.11. Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000. 103:311–320.
Article12. Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis: a look outside the nucleus. Science. 2000. 287:1606–1609.13. Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997. 275:1787–1790.14. Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science. 1993. 262:1731–1734.15. Lam AK. Molecular biology of esophageal squamous cell carcinoma. Crit Rev Oncol Hematol. 2000. 33:71–90.
Article16. McCabe C. Genetic targets for the treatment of pituitary adenomas: focus on the pituitary tumor transforming gene. Curr Opin Pharmacol. 2001. 1:620–625.
Article17. Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002. 4:287–299.
Article18. Armstrong E, Cannizzaro L, Bergman M, Huebner K, Alitalo K. The c-src tyrosine kinase (CSK) gene, a potential antioncogene, localizes to human chromosome region 15q23-25. Cytogenet Cell Genet. 1992. 60:119–120.19. Sherr CJ. Cancer cell cycles. Science. 1996. 274:1672–1677.
Article20. Zhang X, Horwitz GA, Prezant TR, Valentini A, Nakashima M, Bronstein MD, Melmed S. Structure, expression, and function of human pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1999. 13:156–166.
Article21. Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD, Melmed S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999. 84:761–767.
Article22. Dominguez A, Ramos-Morales F, Romero F, Rios RM, Dreyfus F, Tortolero M, Pintor-Toro JA. hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hPTTG. Oncogene. 1998. 17:2187–2193.
Article23. Saez C, Japon MA, Ramos-Morales F, Romero F, Segura DI, Tortolero M, Pintor-Toro JA. hpttg is over-expressed in pituitary adenomas and other primary epithelial neoplasias. Oncogene. 1999. 18:5473–5476.
Article24. Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999. 285:418–422.
Article25. Puri R, Tousson A, Chen L, Kakar SS. Molecular cloning of pituitary tumor transforming gene 1 from ovarian tumors and its expression in tumors. Cancer Lett. 2001. 163:131–139.
Article26. Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001. 11:155–161.
Article27. Myoui A, Nishimura R, Williams PJ, Hiraga T, Tamura D, Michigami T, Mundy GR, Yoneda T. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res. 2003. 63:5028–5033.28. Lim SC, Lee MS. Significance of E-cadherin/beta-catenin complex and cyclin D1 in breast cancer. Oncol Rep. 2002. 9:915–928.29. Brown AM. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 2001. 3:351–355.
Article30. Carthew RW, Rubin GM. Seven in absentia, a gene required for specification R7 cell fate in the Drosophila eye. Cell. 1990. 63:561–577.31. Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White RL, Matsunami N. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001. 7:927–936.
Article32. Matrisian LM, Cunha GR, Mohla S. Epithelial-stromal interactions and tumor progression: meeting summary and future directions. Cancer Res. 2001. 61:3844–3846.33. Curran S, Murray GI. Matrix metalloproteinase: molecular aspects of their roles in tumor invasion and metastasis. Eur J Cancer. 2000. 36:1621–1630.34. Deplanque G, Harris AL. Anti-angiogenic agents: clinical trial design and therapies in development. Eur J Cancer. 2000. 36:1713–1724.35. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of beta-catenin and Adenomatous Polyposis Coli(APC) Protein in Squamous Cell Carcinoma of the Laryngeal Cancers
- Relation between Loss of Heterozygosity of APC gene and: Catenin Expression in Gastric Cancer
- Prognostic Significance of Abnormal beta - catenin Expression in Breast Carcinoma
- Beta-Catenin Downregulation Contributes to Epidermal Growth Factor-induced Migration and Invasion of MDAMB231 Cells
- Correlation of Expression of E-Cadherin, alpha-Catenin, beta-Catenin, and Clinicopathologic Parameters in Colorectal Adenocarcinomas