Endocrinol Metab.
2014 Sep;29(3):336-348. 10.3803/EnM.2014.29.3.336.
Growth Hormone-Releaser Diet Attenuates Cognitive Dysfunction in Klotho Mutant Mice via Insulin-Like Growth Factor-1 Receptor Activation in a Genetic Aging Model
- Affiliations
-
- 1Neuropsychopharmacology and Toxicology Program, Kangwon National University College of Pharmacy, Chunchon, Korea. or shinej@kangwon.ac.kr, kimhc@kangwon.ac.kr
- 2Ilsong Institute of Life Science, Hallym University, Anyang, Korea.
- 3Department of Anatomy, Chung-Ang University College of Medicine, Seoul, Korea.
- 4Department of Pharmacology, Chung-Ang University College of Medicine, Seoul, Korea.
- 5Department of Regional Pharmaceutical Care and Science, Meijo University Graduate School of Pharmaceutical Sciences, Nagoya, Japan.
- KMID: 2169471
- DOI: http://doi.org/10.3803/EnM.2014.29.3.336
Abstract
- BACKGROUND
It has been recognized that a defect in klotho gene expression accelerates the degeneration of multiple age-sensitive traits. Accumulating evidence indicates that aging is associated with declines in cognitive function and the activity of growth hormone (GH)/insulin-like growth factor-1 (IGF-1).
METHODS
In this study, we examined whether a GH-releaser diet could be effective in protecting against cognitive impairment in klotho mutant mice.
RESULTS
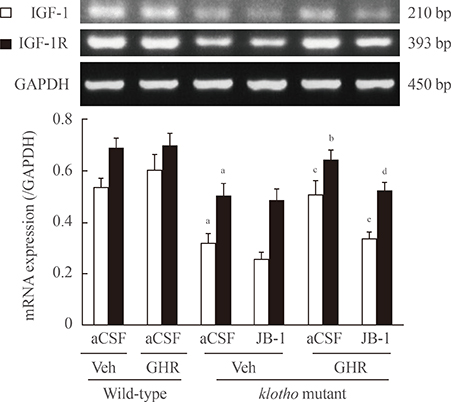
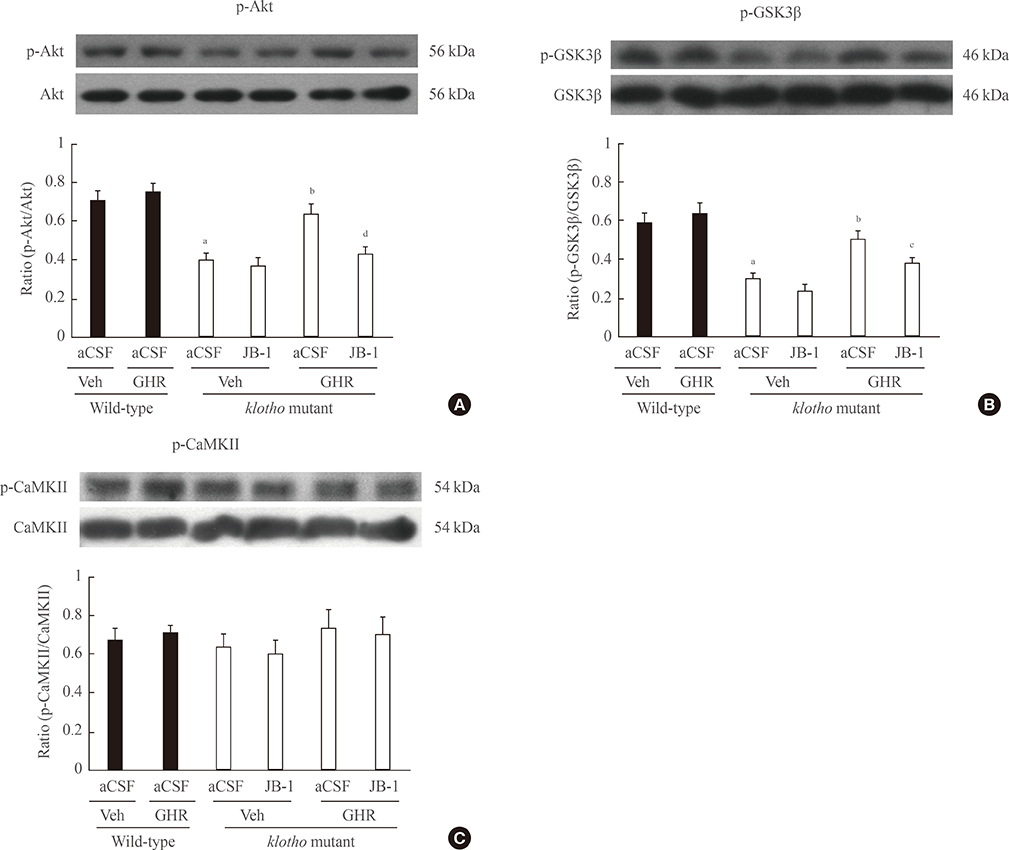
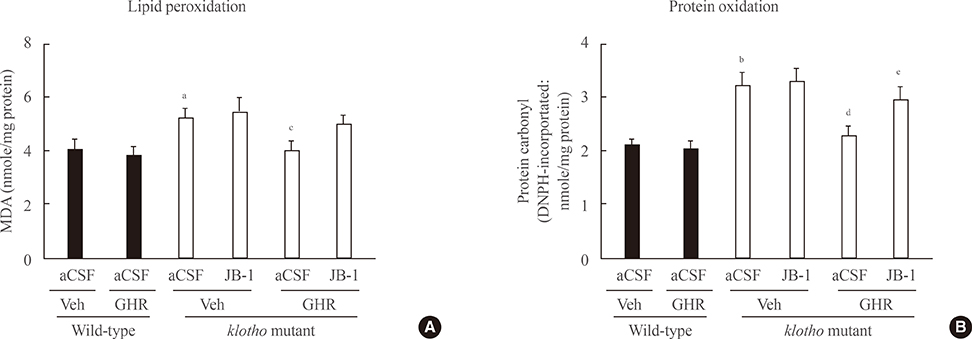
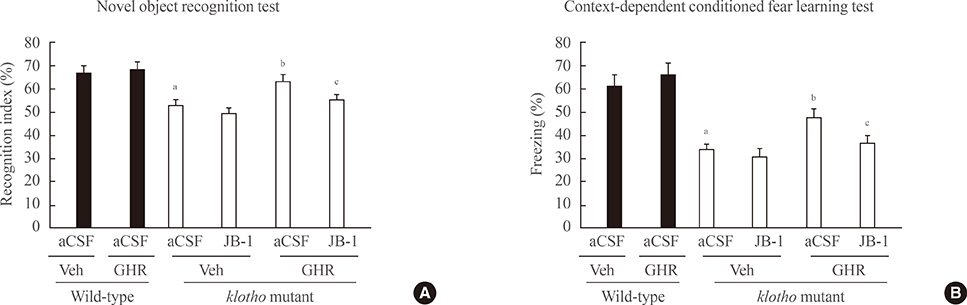
The GH-releaser diet significantly induced the expression of IGF-1 and IGF-1 receptors in the hippocampus of klotho mutant mice. Klotho mutant mice showed significant memory impairments as compared with wild-type mice. In addition, the klotho mutation significantly decreased the expression of cell survival/antiapoptotic factors, including phospho-Akt (p-Akt)/phospho-glycogen synthase kinase3beta (p-GSK3beta), phospho-extracellular signal-related kinase (p-ERK), and Bcl-2, but significantly increased those of cell death/proapoptotic factors, such as phospho-c-jun N-terminal kinase (p-JNK), Bax, and cleaved caspase-3 in the hippocampus. Treatment with GH-releaser diet significantly attenuated both decreases in the expression of cell survival/antiapoptotic factors and increases in the expression of cell death/proapoptotic factors in the hippocampus of klotho mutant mice. In addition, klotho mutation-induced oxidative stress was significantly attenuated by the GH-releaser diet. Consequently, a GH-releaser diet significantly improved memory function in the klotho mutant mice. GH-releaser diet-mediated actions were significantly reversed by JB-1, an IGF-1 receptor antagonist.
CONCLUSION
The results suggest that a GH-releaser diet attenuates oxidative stress, proapoptotic changes and consequent dysfunction in klotho mutant mice by promoting IGF-1 expression and IGF-1 receptor activation.
MeSH Terms
Figure
Reference
-
1. Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998; 242:626–630.2. Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998; 424:6–10.3. Kuro-o M. Klotho and aging. Biochim Biophys Acta. 2009; 1790:1049–1058.4. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390:45–51.5. Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006; 15:437–441.6. Kuro-o M. Klotho. Pflugers Arch. 2010; 459:333–343.7. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005; 309:1829–1833.8. Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008; 389:233–241.9. Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003; 17:50–52.10. Anamizu Y, Kawaguchi H, Seichi A, Yamaguchi S, Kawakami E, Kanda N, Matsubara S, Kuro-o M, Nabeshima Y, Nakamura K, Oyanagi K. Klotho insufficiency causes decrease of ribosomal RNA gene transcription activity, cytoplasmic RNA and rough ER in the spinal anterior horn cells. Acta Neuropathol. 2005; 109:457–466.11. Park SJ, Shin EJ, Min SS, An J, Li Z, Hee Chung Y, Hoon Jeong J, Bach JH, Nah SY, Kim WK, Jang CG, Kim YS, Nabeshima Y, Nabeshima T, Kim HC. Inactivation of JAK2/STAT3 signaling axis and downregulation of M1 mAChR cause cognitive impairment in klotho mutant mice, a genetic model of aging. Neuropsychopharmacology. 2013; 38:1426–1437.12. Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004; 29:91–99.13. Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997; 278:419–424.14. van Dam PS, Aleman A, de Vries WR, Deijen JB, van der Veen EA, de Haan EH, Koppeschaar HP. Growth hormone, insulin-like growth factor I and cognitive function in adults. Growth Horm IGF Res. 2000; 10:Suppl B. S69–S73.15. Khorram O. Use of growth hormone and growth hormone secretagogues in aging: help or harm. Clin Obstet Gynecol. 2001; 44:893–901.16. Chromiak JA, Antonio J. Use of amino acids as growth hormone-releasing agents by athletes. Nutrition. 2002; 18:657–661.17. Shin EJ, Jhoo JH, Nabeshima T, Jhoo WK, Kwon MS, Lim YK, Chae JS, Jung ME, Park SJ, Jang KJ, Kim HC. Growth hormone releaser attenuates beta-amyloid (1 - 42)-induced memory impairment in mice. J Pharmacol Sci. 2005; 99:117–120.18. Shin EJ, Chae JS, Park SJ, Kim SC, Koo KH, Yamada K, Nabeshima T, Kim HC. Growth hormone-releaser diet attenuates beta-amyloid(1-42)-induced cognitive impairment via stimulation of the insulin-like growth factor (IGF)-1 receptor in mice. J Pharmacol Sci. 2009; 109:139–143.19. Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987; 262:5488–5491.20. Tran HY, Shin EJ, Saito K, Nguyen XK, Chung YH, Jeong JH, Bach JH, Park DH, Yamada K, Nabeshima T, Yoneda Y, Kim HC. Protective potential of IL-6 against trimethyltin-induced neurotoxicity in vivo. Free Radic Biol Med. 2012; 52:1159–1174.21. Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, Aizawa H, Itoh H, Kurabayashi M, Kawazu S, Tomono S, Oka Y, Suga T, Kuro-o M, Nabeshima Y, Nagai R. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism. 2000; 49:1118–1123.22. Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008; 27:7094–7105.23. Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005; 280:38029–38034.24. Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007; 104:19796–19801.25. Lorenzi O, Veyrat-Durebex C, Wollheim CB, Villemin P, Rohner-Jeanrenaud F, Zanchi A, Vischer UM. Evidence against a direct role of klotho in insulin resistance. Pflugers Arch. 2010; 459:465–473.26. Frago LM, Paneda C, Dickson SL, Hewson AK, Argente J, Chowen JA. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology. 2002; 143:4113–4122.27. Locatelli V, Rossoni G, Schweiger F, Torsello A, De Gennaro Colonna V, Bernareggi M, Deghenghi R, Muller EE, Berti F. Growth hormone-independent cardioprotective effects of hexarelin in the rat. Endocrinology. 1999; 140:4024–4031.28. Tivesten A, Bollano E, Caidahl K, Kujacic V, Sun XY, Hedner T, Hjalmarson A, Bengtsson BA, Isgaard J. The growth hormone secretagogue hexarelin improves cardiac function in rats after experimental myocardial infarction. Endocrinology. 2000; 141:60–66.29. Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002; 159:1029–1037.30. Pang JJ, Xu RK, Xu XB, Cao JM, Ni C, Zhu WL, Asotra K, Chen MC, Chen C. Hexarelin protects rat cardiomyocytes from angiotensin II-induced apoptosis in vitro. Am J Physiol Heart Circ Physiol. 2004; 286:H1063–H1069.31. English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996; 271:24329–24332.32. Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998; 1:602–609.33. Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007; 14:117–125.34. Tong L, Balazs R, Thornton PL, Cotman CW. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004; 24:6799–6809.35. Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006; 13:349–358.36. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995; 378:785–789.37. Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997; 17:1595–1606.38. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998; 273:19929–19932.39. Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci U S A. 2002; 99:7951–7955.40. Kashimada K, Yamashita T, Tsuji K, Nifuji A, Mizutani S, Nabeshima Y, Noda M. Defects in growth and bone metabolism in klotho mutant mice are resistant to GH treatment. J Endocrinol. 2002; 174:403–410.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship of Insulin like Growth Factor I with Pharmacologically Stimulated Growth Hormone Secretion in Growth Hormone Deficient Children
- Klotho and the Aging Process
- Expression Pattern and Role of Klotho in Human Hair Follicles
- Changes in Growth Hormone-Axis Function in Nutrient Excess or Deprivation
- Antiaging Efforts in Endocrine Aspects