Endocrinol Metab.
2014 Jun;29(2):179-184. 10.3803/EnM.2014.29.2.179.
Gene Expression Regulation by Agonist-Independent Constitutive Signaling of Melanocortin-1 Receptor
- Affiliations
-
- 1Department of Life Science and Ewha Research Center for Systems Biology, Ewha Womans University, Seoul, Korea. jkim1964@ewha.ac.kr
- KMID: 2169425
- DOI: http://doi.org/10.3803/EnM.2014.29.2.179
Abstract
- BACKGROUND
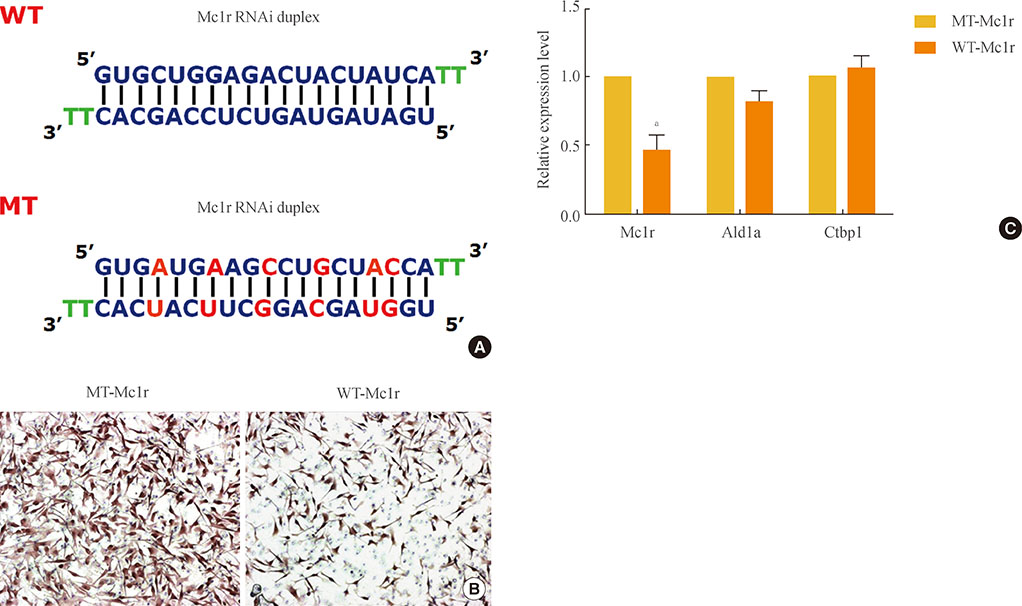
Melanocortin-1 receptor (Mc1r), a key signaling receptor for melanogenesis, has been reported to mediate migration of B16F10 melanoma cells. Interestingly, this activity appears to be a part of the constitutive signaling of Mc1r.
METHODS
We carried out small interfering RNA-mediated knock-down of Mc1r on murine melanoma B16F10 cells and performed microarray analysis to characterize changes in the gene expression profile.
RESULTS
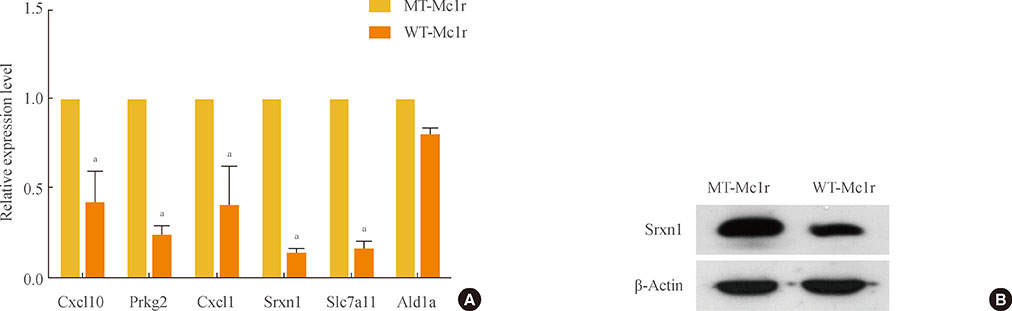
We isolated 22 and four genes whose expression decreased and increased, respectively, by 2.5-fold or higher as the result of Mc1r knock-down. Several down-regulated genes have been proposed to be involved in cell migration. Among these genes are several members of the chemokine gene family.
CONCLUSION
We provide a gene set for further functional analyses of Mc1r. The Mc1r target genes we present may be particularly relevant for understanding the ligand-independent activity of Mc1r. Further examination of the mode of action may lead to novel strategies in regulating the migration and metastasis of melanoma cells.
MeSH Terms
Figure
Reference
-
1. Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005; 18:393–410.2. Le Pape E, Passeron T, Giubellino A, Valencia JC, Wolber R, Hearing VJ. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proc Natl Acad Sci U S A. 2009; 106:1802–1807.3. Murata J, Ayukawa K, Ogasawara M, Fujii H, Saiki I. Alpha-melanocyte-stimulating hormone blocks invasion of reconstituted basement membrane (Matrigel) by murine B16 melanoma cells. Invasion Metastasis. 1997; 17:82–93.4. Liu GS, Liu LF, Lin CJ, Tseng JC, Chuang MJ, Lam HC, Lee JK, Yang LC, Chan JH, Howng SL, Tai MH. Gene transfer of pro-opiomelanocortin prohormone suppressed the growth and metastasis of melanoma: involvement of alpha-melanocyte-stimulating hormone-mediated inhibition of the nuclear factor kappaB/cyclooxygenase-2 pathway. Mol Pharmacol. 2006; 69:440–451.5. Seong I, Min HJ, Lee JH, Yeo CY, Kang DM, Oh ES, Hwang ES, Kim J. Sox10 controls migration of B16F10 melanoma cells through multiple regulatory target genes. PLoS One. 2012; 7:e31477.6. Wei Q, Jiang H, Xiao Z, Baker A, Young MR, Veenstra TD, Colburn NH. Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc Natl Acad Sci U S A. 2011; 108:7004–7009.7. Bowers RR, Manevich Y, Townsend DM, Tew KD. Sulfiredoxin redox-sensitive interaction with S100A4 and non-muscle myosin IIA regulates cancer cell motility. Biochemistry. 2012; 51:7740–7754.8. Gao SP, Bromberg JF. Touched and moved by STAT3. Sci STKE. 2006; 2006:pe30.9. Teng TS, Lin B, Manser E, Ng DC, Cao X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J Cell Sci. 2009; 122(Pt 22):4150–4159.10. Long H, Xie R, Xiang T, Zhao Z, Lin S, Liang Z, Chen Z, Zhu B. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells. 2012; 30:2309–2319.11. Wang SW, Wu HH, Liu SC, Wang PC, Ou WC, Chou WY, Shen YS, Tang CH. CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PLoS One. 2012; 7:e35101.12. Isgro M, Bianchetti L, Marini MA, Bellini A, Schmidt M, Mattoli S. The C-C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol. 2013; 6:718–727.13. Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002; 118:915–922.14. Richmond A, Thomas HG. Melanoma growth stimulatory activity: isolation from human melanoma tumors and characterization of tissue distribution. J Cell Biochem. 1988; 36:185–198.15. Di Cesare S, Marshall JC, Logan P, Antecka E, Faingold D, Maloney SC, Burnier MN Jr. Expression and migratory analysis of 5 human uveal melanoma cell lines for CXCL12, CXCL8, CXCL1, and HGF. J Carcinog. 2007; 6:2.16. Woodward JK, Elshaw SR, Murray AK, Nichols CE, Cross N, Laws D, Rennie IG, Sisley K. Stimulation and inhibition of uveal melanoma invasion by HGF, GRO, IL-1alpha and TGF-beta. Invest Ophthalmol Vis Sci. 2002; 43:3144–3152.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic obesity: an update with emerging therapeutic approaches
- SR144528 as Inverse Agonist of CB2 Cannabinoid Receptor
- Melanocortin 4 Receptor and Dopamine D2 Receptor Expression in Brain Areas Involved in Food Intake
- The Expression of the alpha-Melanocyte Stimulating Hormone (alpha-MSH) and Melanocortin-1 Receptor (MC1R) in the Epidermis of the Vitiligo
- Role of angiotensin II and nitric oxide in the rat paraventricular nucleus