Ann Pediatr Endocrinol Metab.
2022 Sep;27(3):169-175. 10.6065/apem.2244188.094.
Genetic obesity: an update with emerging therapeutic approaches
- Affiliations
-
- 1Department of Medical Genetics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- KMID: 2533335
- DOI: http://doi.org/10.6065/apem.2244188.094
Abstract
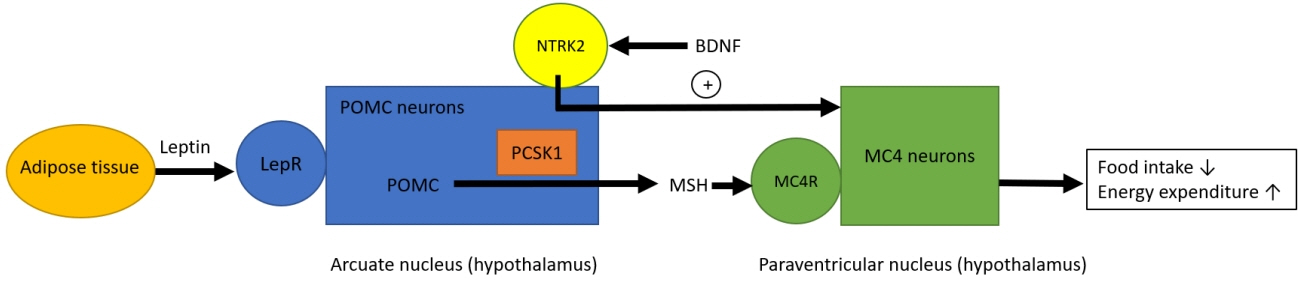
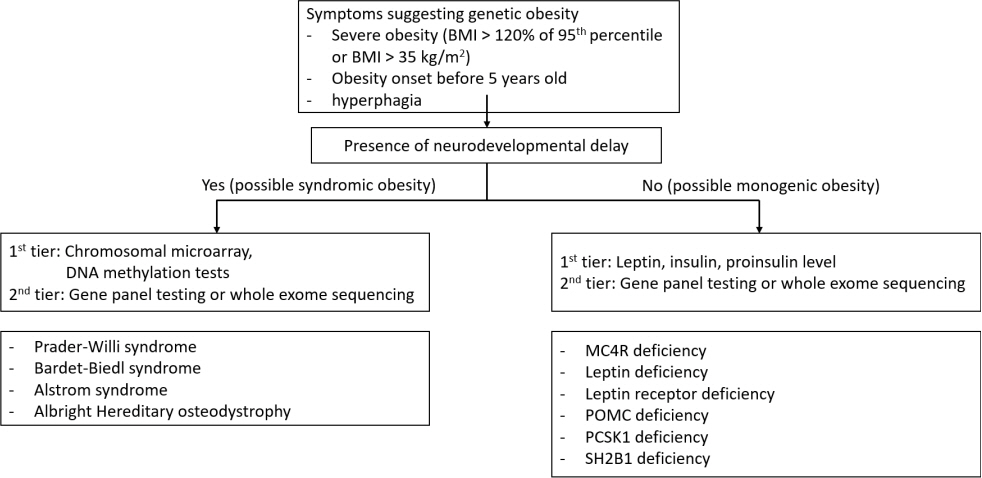
- Based on the genetic contribution, childhood obesity can be classified into 3 groups: common polygenic obesity, syndromic obesity, and monogenic obesity. More genetic causes of obesity are being identified along with the advances in the genetic testing. Genetic obesities including syndromic and monogenic obesity should be suspected and evaluated in children with early-onset morbid obesity and hyperphagia under 5 years of age. Patients with syndromic obesity have early-onset severe obesity associated specific genetic syndromes including Prader-Willi syndrome, Bardet-Biedle syndrome, and Alstrom syndrome. Syndromic obesity is often accompanied with neurodevelopmental delay or dysmorphic features. Nonsyndromic monogenic obesity is caused by variants in single gene which are usually involved in the regulation of hunger and satiety associated with the hypothalamic leptin-melanocortin pathway in central nervous system. Unlike syndromic obesity, patients with monogenic obesity usually show normal neurodevelopment. They would be presented with hyperphagia and early-onset severe obesity with additional clinical symptoms including short stature, red hair, adrenal insufficiency, hypothyroidism, hypogonadism, pituitary insufficiencies, diabetes insipidus, increased predisposition to infection or intractable recurrent diarrhea. Identifying patients with genetic obesity is critical as new innovative therapies including melanocortin 4 receptor agonist have become available. Early genetic evaluation enables to identify treatable obesity and provide timely intervention which may eventually achieve favorable outcome by establishing personalized management.
Figure
Reference
-
References
1. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017; 102:709–57.2. Moreno LA. Obesity: early severe obesity in children. Nat Rev Endocrinol. 2018; 14:194–6.3. Hinney A, Körner A, Fischer-Posovszky P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat Rev Endocrinol. 2022; 18:623–37.4. Landgraf K, Rockstroh D, Wagner IV, Weise S, Tauscher R, Schwartze JT, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesityrelated inflammation and insulin resistance in children. Diabetes. 2015; 64:1249–61.5. Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016; 374:2430–40.6. Dayton K, Miller J. Finding treatable genetic obesity: strategies for success. Curr Opin Pediatr. 2018; 30:526–31.7. Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev. 2017; 18:603–34.8. Poitou C, Mosbah H, Clément K. Mechanisms in endocrinology: update on treatments for patients with genetic obesity. Eur J Endocrinol. 2020; 183:R149–66.9. Lloret-Linares C, Faucher P, Coupaye M, Alili R, Green A, Basdevant A, et al. Comparison of body composition, basal metabolic rate and metabolic outcomes of adults with Prader Willi syndrome or lesional hypothalamic disease, with primary obesity. Int J Obes (Lond). 2013; 37:1198–203.10. Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008; 93:4183–97.11. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012; 14:10–26.12. Kim SJ, Cho SY, Jin DK. Prader-Willi syndrome: an update on obesity and endocrine problems. Ann Pediatr Endocrinol Metab. 2021; 26:227–36.13. Kim YM, Lee YJ, Kim SY, Cheon CK, Lim HH. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader-Willi syndrome. Ann Pediatr Endocrinol Metab. 2020; 25:52–6.14. Tsang SH, Aycinena ARP, Sharma T. Ciliopathy: Bardet-Biedl syndrome. Adv Exp Med Biol. 2018; 1085:171–4.15. Chennen K, Scerbo MJ, Dollfus H, Poch O, Marion V. Syndrome de Bardet-Biedl: cils et obésité - de la génétique aux approches intégratives Bardet-Biedl syndrome: cilia and obesity - from genes to integrative approaches. Med Sci (Paris). 2014; 30:1034–9.16. Haq N, Schmidt-Hieber C, Sialana FJ, Ciani L, Heller JP, Stewart M, et al. Loss of Bardet-Biedl syndrome proteins causes synaptic aberrations in principal neurons. PLoS Biol. 2019; 17:e3000414.17. Haws R, Brady S, Davis E, Fletty K, Yuan G, Gordon G, et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020; 22:2133–40.18. Haws RM, Gordon G, Han JC, Yanovski JA, Yuan G, Stewart MW. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: Phase 3 trial design. Contemp Clin Trials Commun. 2021; 22:100780.19. Marshall JD, Beck S, Maffei P, Naggert JK. Alström syndrome. Eur J Hum Genet. 2007; 15:1193–202.20. Marshall JD, Muller J, Collin GB, Milan G, Kingsmore SF, Dinwiddie D, et al. Alström syndrome: mutation spectrum of ALMS1. Hum Mutat. 2015; 36:660–8.21. Haldeman-Englert CR, Hurst ACE, Levine MA. Disorders of GNAS inactivation. In : Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, editors. GeneReviews(®). Seattle (WA): University of Washington, Seattle;1993.22. Mendes de Oliveira E, Keogh JM, Talbot F, Henning E, Ahmed R, Perdikari A, et al. Obesity-associated GNAS mutations and the melanocortin pathway. N Engl J Med. 2021; 385:1581–92.23. Farooqi IS. Monogenic human obesity syndromes. Handb Clin Neurol. 2021; 181:301–10.24. Lubrano-Berthelier C, Dubern B, Lacorte JM, Picard F, Shapiro A, Zhang S, et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab. 2006; 91:1811–8.25. Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008; 4:569–77.26. Nordang GBN, Busk ØL, Tveten K, Hanevik HI, Fell AKM, Hjelmesæth J, et al. Next-generation sequencing of the monogenic obesity genes LEP, LEPR, MC4R, PCSK1 and POMC in a Norwegian cohort of patients with morbid obesity and normal weight controls. Mol Genet Metab. 2017; 121:51–6.27. Wasim M, Awan FR, Najam SS, Khan AR, Khan HN. Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem Genet. 2016; 54:565–72.28. Funcke JB, von Schnurbein J, Lennerz B, Lahr G, Debatin KM, Fischer-Posovszky P, et al. Monogenic forms of childhood obesity due to mutations in the leptin gene. Mol Cell Pediatr. 2014; 1:3.29. Kleinendorst L, Abawi O, van der Kamp HJ, Alders M, Meijers-Heijboer HEJ, van Rossum EFC, et al. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. Eur J Endocrinol. 2020; 182:47–56.30. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007; 356:237–47.31. Yeo GSH, Chao DHM, Siegert AM, Koerperich ZM, Ericson MD, Simonds SE, et al. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol Metab. 2021; 48:101206.32. Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E, et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab. 2007; 92:3369–73.33. Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997; 16:303–6.34. Li Z, Zhou Y, Carter-Su C, Myers MG Jr, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol. 2007; 21:2270–81.35. Rui L. SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J Diabetes. 2014; 5:511–26.36. Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012; 122:4732–6.37. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999; 341:879–84.38. de Candia P, Prattichizzo F, Garavelli S, Alviggi C, La Cava A, Matarese G. The pleiotropic roles of leptin in metabolism, immunity, and cancer. J Exp Med. 2021; 218:e20191593.39. Kühnen P, Clément K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016; 375:240–6.40. Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020; 8:960–70.41. Markham A. Setmelanotide: first approval. Drugs. 2021; 81:397–403.42. Macneil DJ. The role of melanin-concentrating hormone and its receptors in energy homeostasis. Front Endocrinol (Lausanne). 2013; 4:49.43. Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, et al. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006; 55:2549–53.44. Angelidi AM, Belanger MJ, Kokkinos A, Koliaki CC, Mantzoros CS. Novel noninvasive approaches to the treatment of obesity: from pharmacotherapy to gene therapy. Endocr Rev. 2022; 43:507–57.45. Yamanaka S. Pluripotent stem cell-based cell therapypromise and challenges. Cell Stem Cell. 2020; 27:523–31.46. Uddin F, Rudin CM, Sen T. CRISPR gene therapy: applications, limitations, and implications for the future. Front Oncol. 2020; 10:1387.